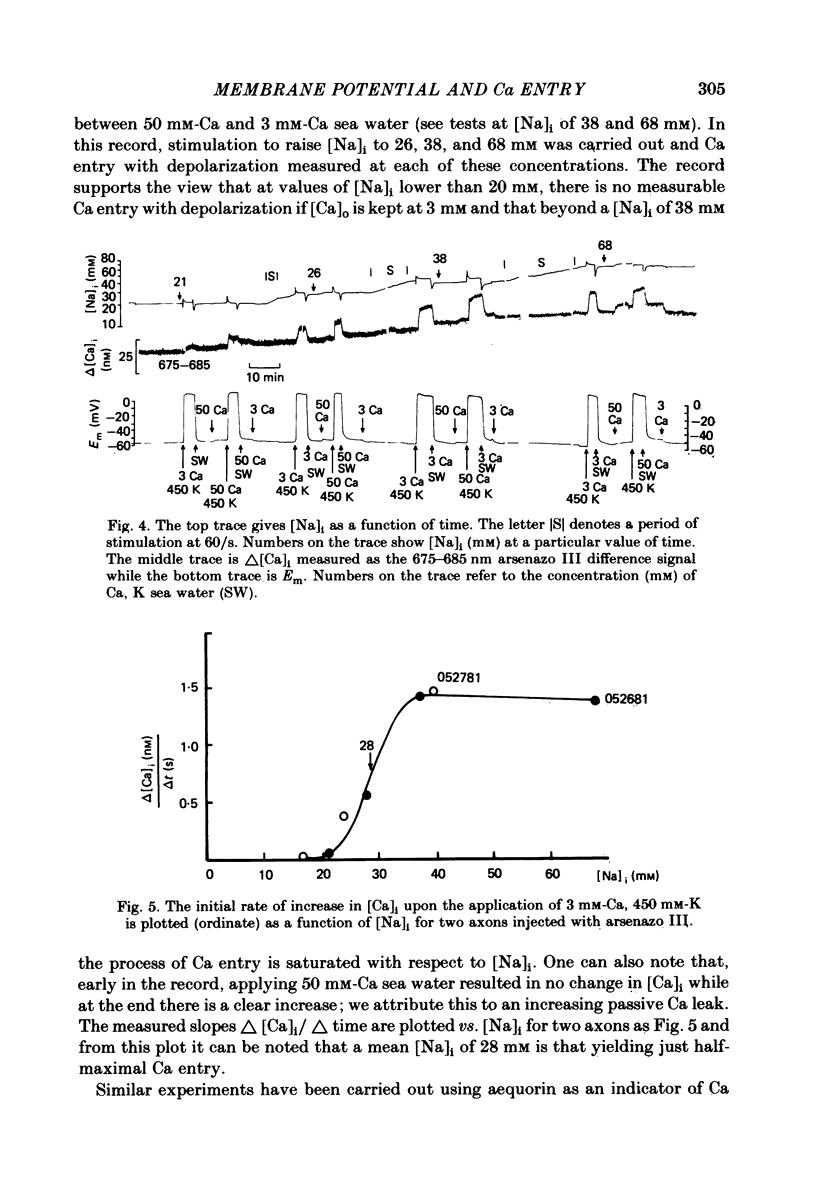
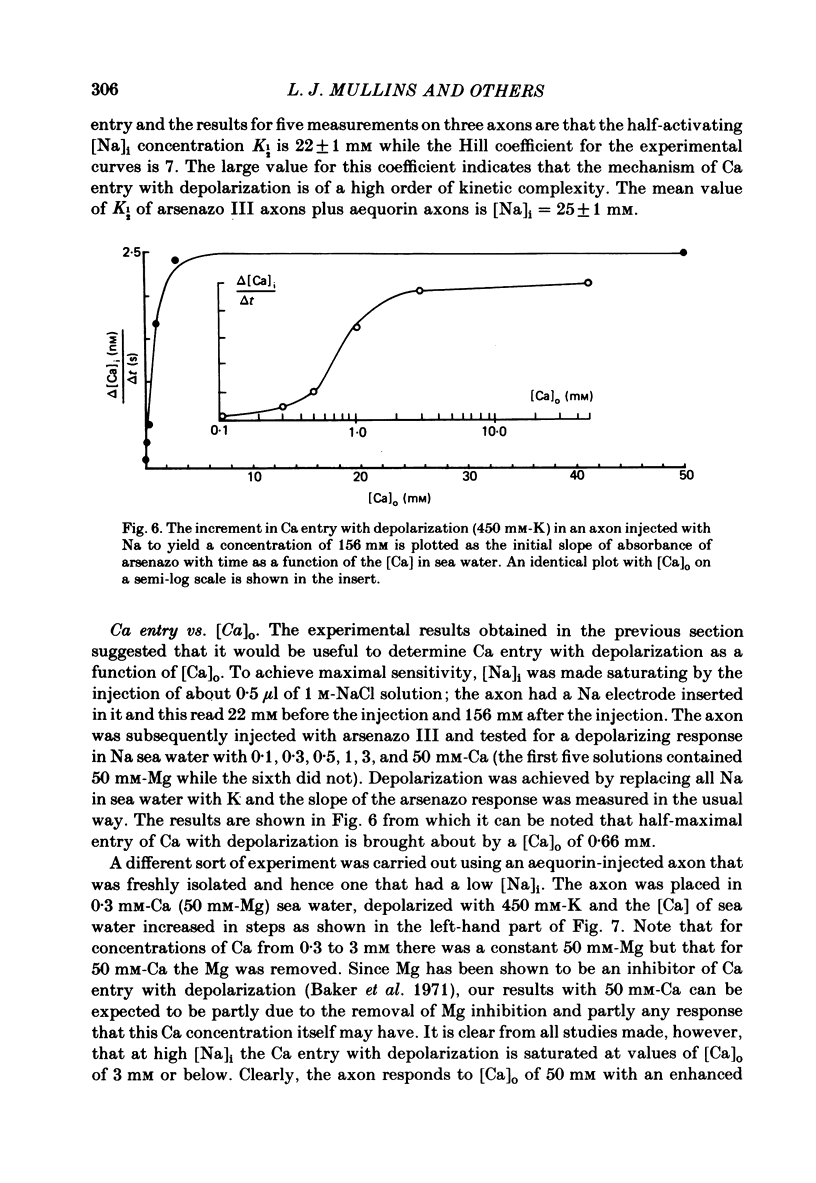
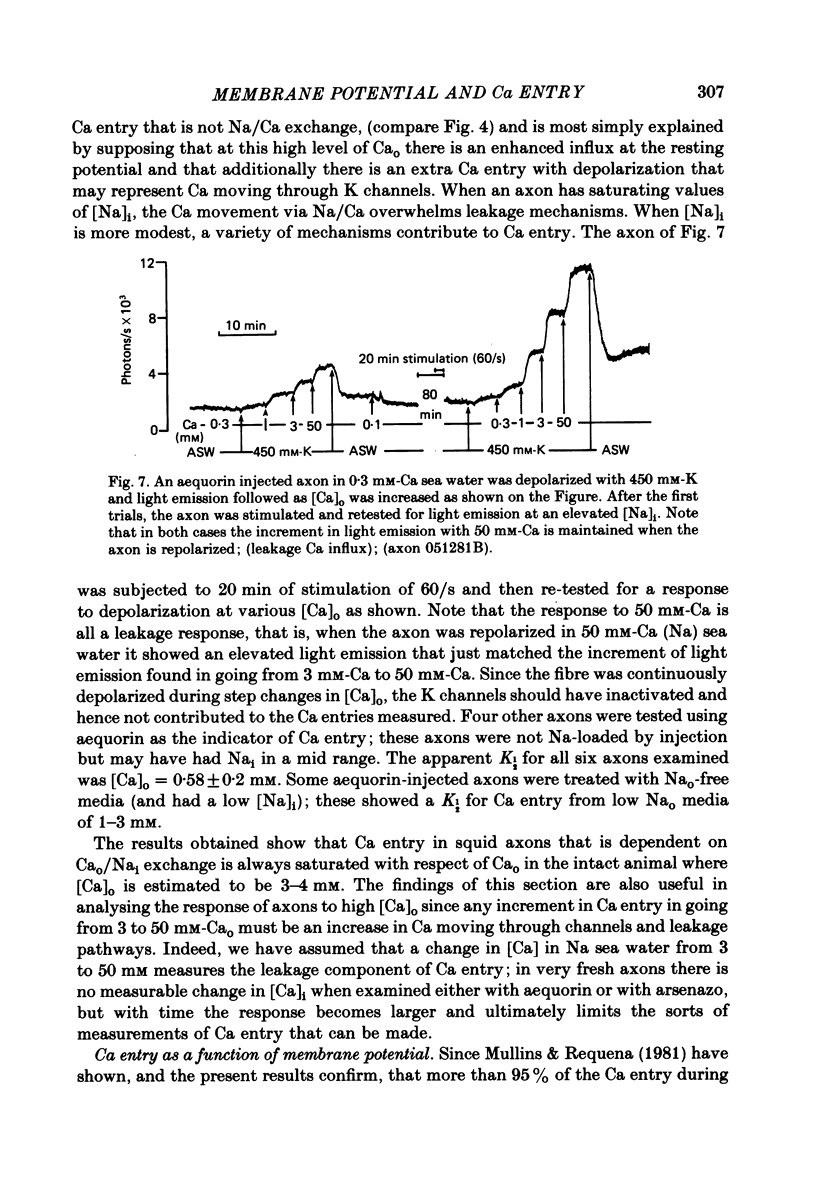
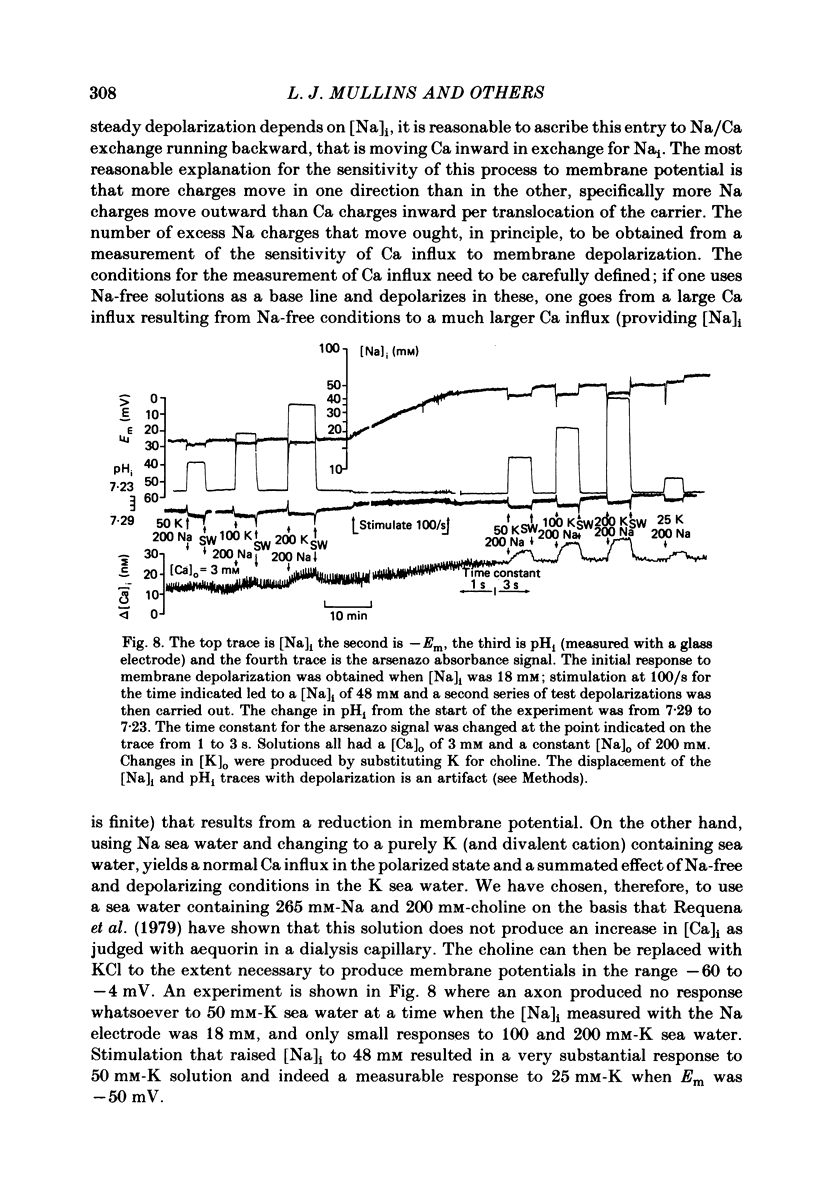
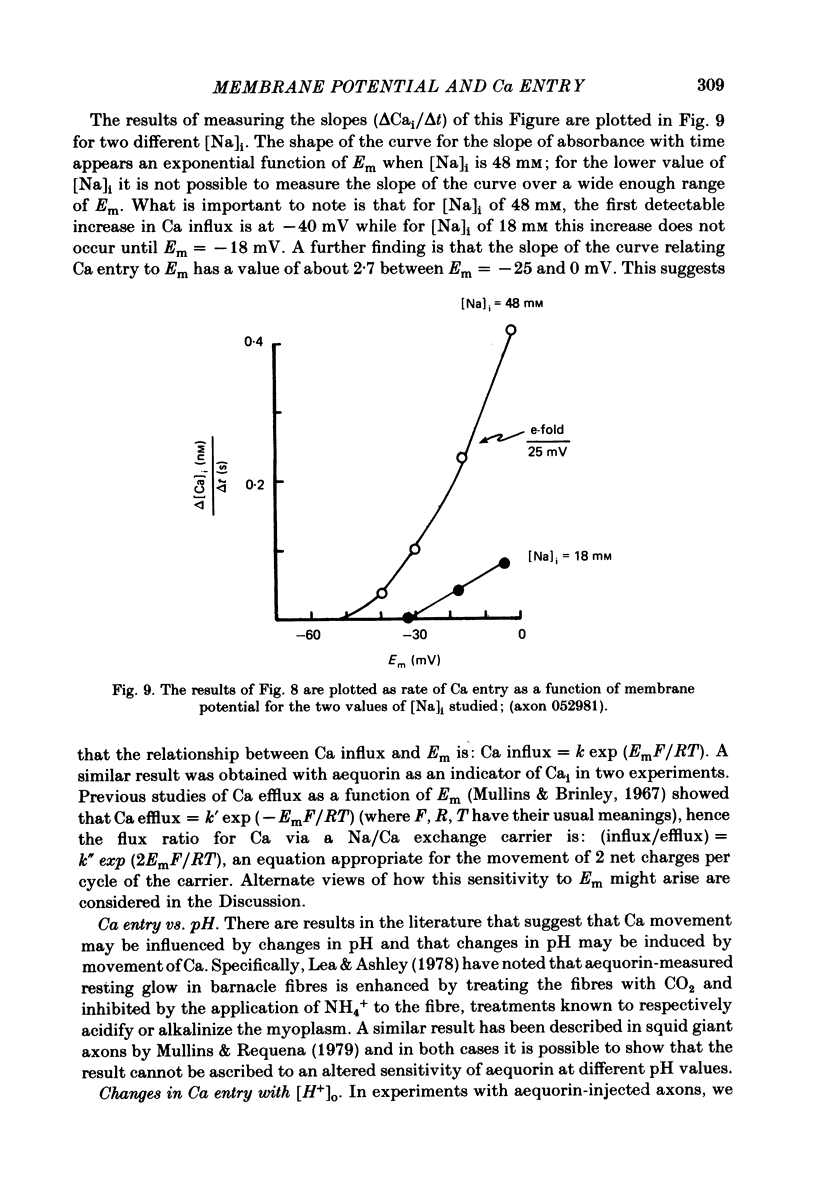
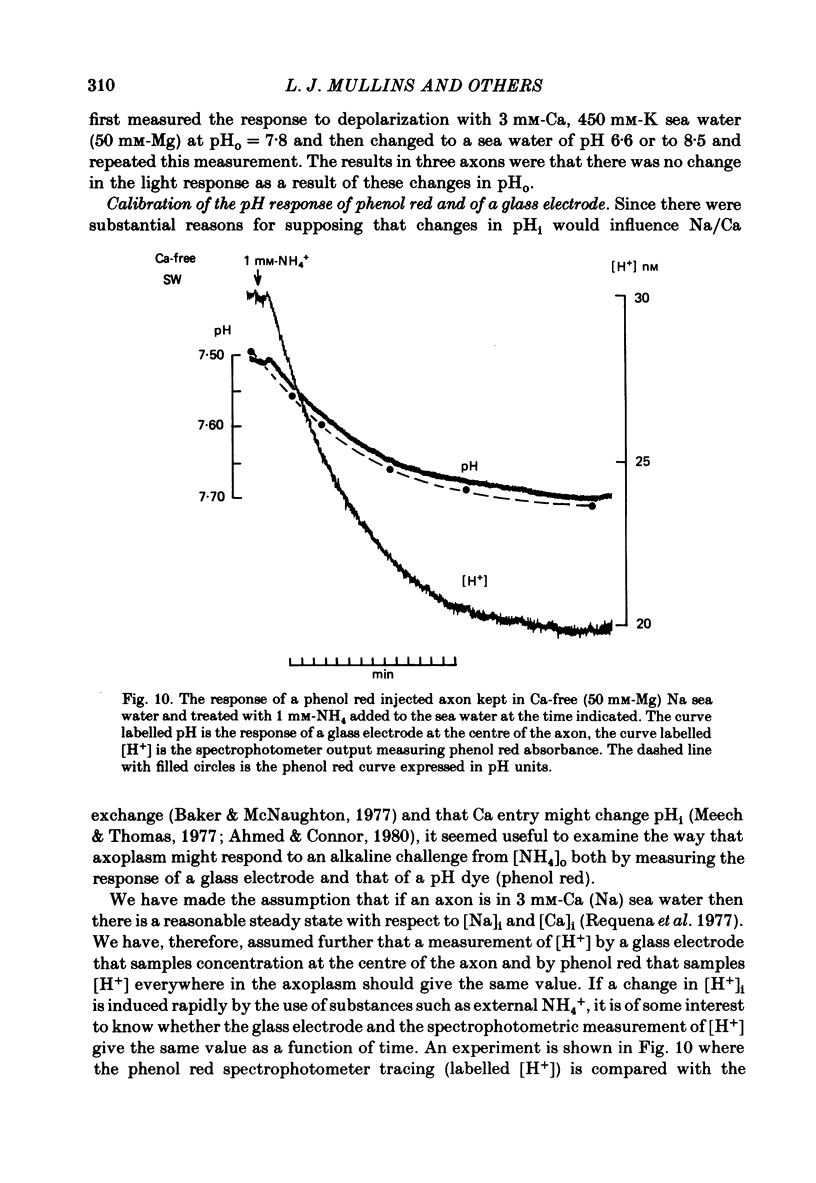
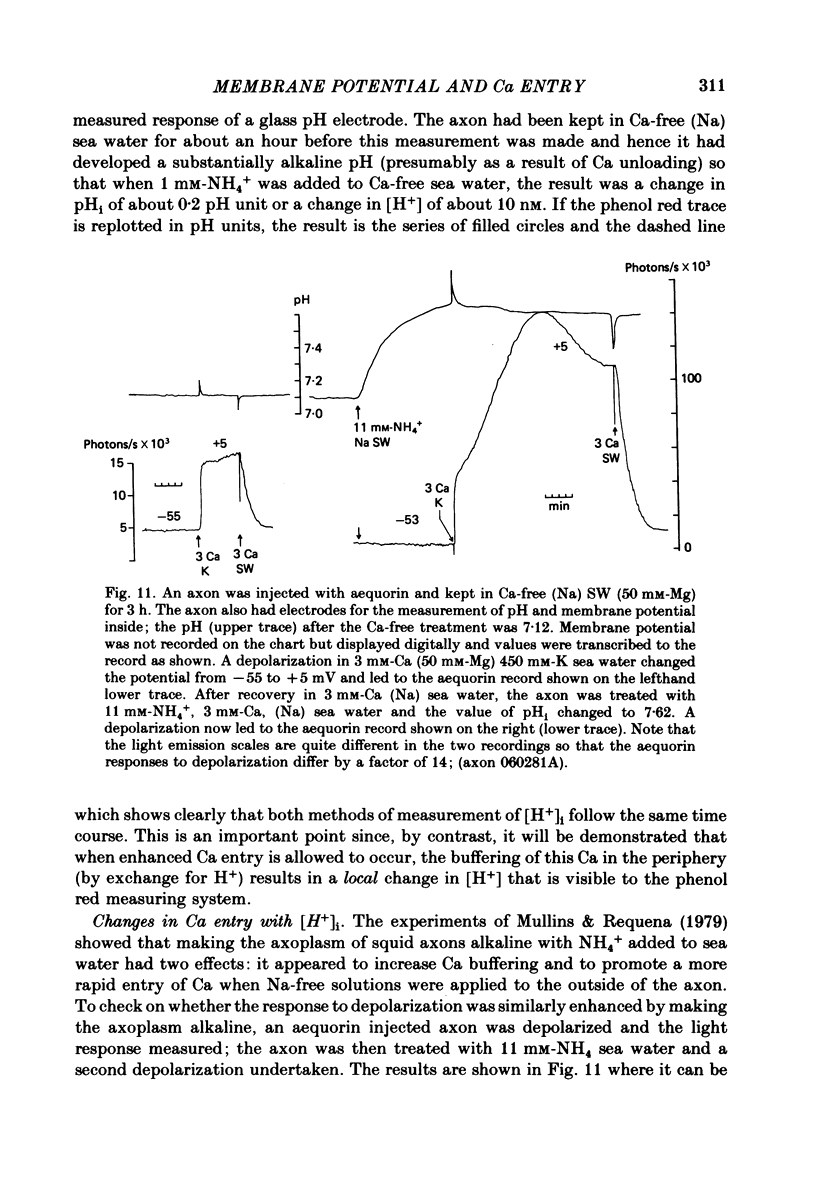
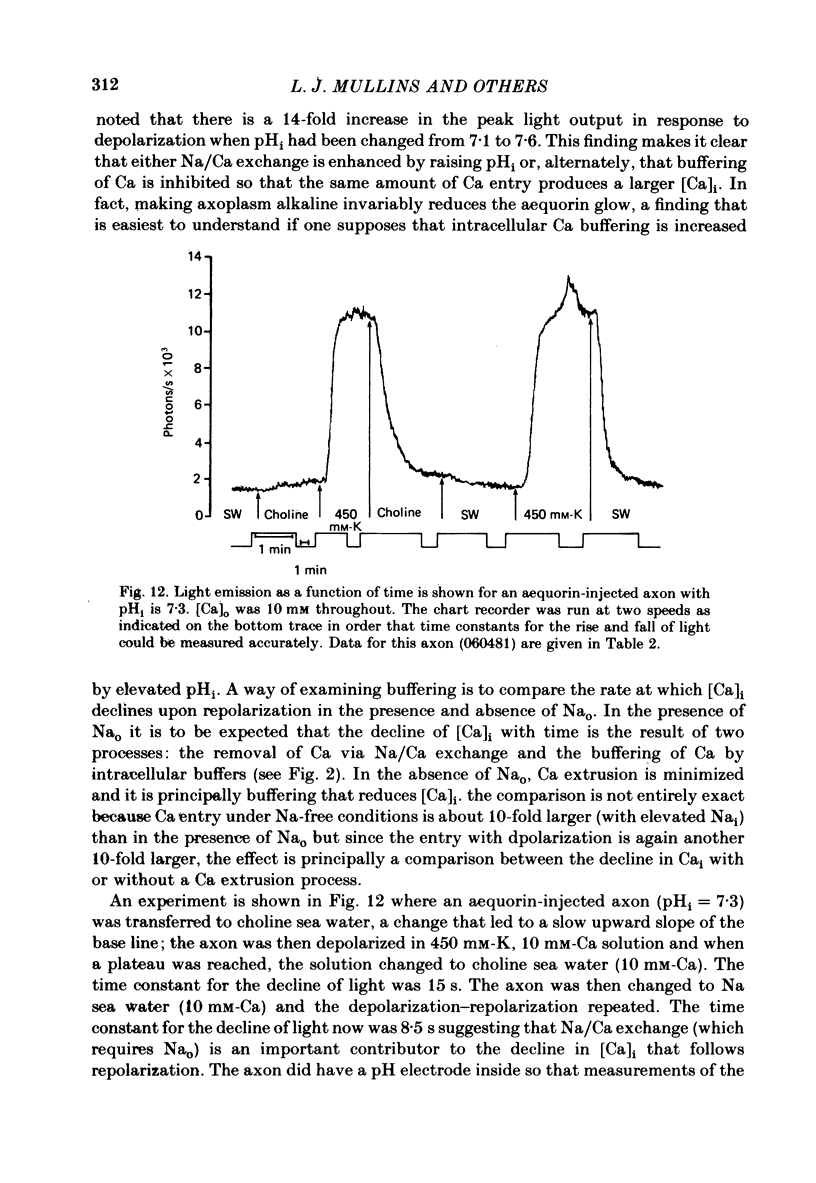
Abstract
Squid giant axons were impaled with electrodes to measure pNai, pHi, Em, and were injected with either aequorin or arsenazo III to measure [Ca]i or with phenol red to measure [H]i. Depolarization of such axons with elevated [K] in sea water leads to a Ca entry that is a function of [Ca]o, [Na]i, and [H]i. With saturating [Na]i half-maximal Ca entry is produced by a [Ca]o of 0.58 mM. With saturating [Ca]o, depolarization produced by 450 mM-K+ leads to half-maximal Ca entry when [Na]i is 25 mM; entry is virtually undetectable if [Na]i is 18 mM. If [Ca]o is 50 mM, Ca entry upon depolarization as measured with aequorin is phasic with a rapid phase of light emission and a plateau; Ca entry as measured with arsenazo III shows no such phasic behaviour, absorbance vs. time is a square wave that closely follows the depolarization vs. time trace. Both detectors of [Ca]i show a square-wave response if [Ca]o is 3 mM. The introduction of 2 mM-CN into the sea water bathing the axon does not affect the response to depolarization nor does the destruction of most of the ATP in the axon following the injection of apyrase. If axons are microinjected with phenol red rather than arsenazo, the entry of Ca produces an acidification in the peripheral parts of the axoplasm. Other experiments measuring [Ca]i show that Ca entry is strongly inhibited by a decrease in pHi. Making sea water alkaline with pH buffers scarcely affects the Ca entry induced by depolarization; making axoplasm alkaline by adding NH4+ to sea water greatly enhances Ca entry by Na/Ca exchange and also enhances the ability of axoplasmic buffers to absorb Ca.
Full text
PDF









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahmed Z., Connor J. A. Intracellular pH changes induced by calcium influx during electrical activity in molluscan neurons. J Gen Physiol. 1980 Apr;75(4):403–426. doi: 10.1085/jgp.75.4.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed Z., Kragie L., Connor J. A. Stoichiometry and apparent dissociation constant of the calcium-arsenazo III reaction under physiological conditions. Biophys J. 1980 Dec;32(3):907–920. doi: 10.1016/S0006-3495(80)85025-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker P. F., Blaustein M. P., Hodgkin A. L., Steinhardt R. A. The influence of calcium on sodium efflux in squid axons. J Physiol. 1969 Feb;200(2):431–458. doi: 10.1113/jphysiol.1969.sp008702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker P. F., Honerjäger P. Influence of carbon dioxide on level of ionised calcium in squid axons. Nature. 1978 May 11;273(5658):160–161. doi: 10.1038/273160a0. [DOI] [PubMed] [Google Scholar]
- Baker P. F., McNaughton P. A. Kinetics and energetics of calcium efflux from intact squid giant axons. J Physiol. 1976 Jul;259(1):103–144. doi: 10.1113/jphysiol.1976.sp011457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker P. F., McNaughton P. A. Selective inhibition of the Ca-dependent Na efflux from intact squid axons by a fall in intracellular pH [proceedings]. J Physiol. 1977 Jul;269(1):78P–79P. [PubMed] [Google Scholar]
- Baker P. F., Meves H., Ridgway E. B. Calcium entry in response to maintained depolarization of squid axons. J Physiol. 1973 Jun;231(3):527–548. doi: 10.1113/jphysiol.1973.sp010247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker P. F., Meves H., Ridgway E. B. Effects of manganese and other agents on the calcium uptake that follows depolarization of squid axons. J Physiol. 1973 Jun;231(3):511–526. doi: 10.1113/jphysiol.1973.sp010246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boron W. F., De Weer P. Intracellular pH transients in squid giant axons caused by CO2, NH3, and metabolic inhibitors. J Gen Physiol. 1976 Jan;67(1):91–112. doi: 10.1085/jgp.67.1.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinley F. J., Jr, Mullins L. J. Sodium extrusion by internally dialyzed squid axons. J Gen Physiol. 1967 Nov;50(10):2303–2331. doi: 10.1085/jgp.50.10.2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinley F. J., Jr, Spangler S. G., Mullins L. J. Calcium and EDTA fluxes in dialyzed squid axons. J Gen Physiol. 1975 Aug;66(2):223–250. doi: 10.1085/jgp.66.2.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinley F. J., Jr, Tiffert T., Scarpa A. Mitochondria and other calcium buffers of squid axon studied in situ. J Gen Physiol. 1978 Jul;72(1):101–127. doi: 10.1085/jgp.72.1.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinley F. J., Jr, Tiffert T., Scarpa A., Mullins L. J. Intracellular calcium buffering capacity in isolated squid axons. J Gen Physiol. 1977 Sep;70(3):355–384. doi: 10.1085/jgp.70.3.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown J. E., Cohen L. B., De Weer P., Pinto L. H., Ross W. N., Salzberg B. M. Rapid changes in intracellular free calcium concentration. Detection by metallochromic indicator dyes in squid giant axon. Biophys J. 1975 Nov;15(11):1155–1160. doi: 10.1016/S0006-3495(75)85891-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CALDWELL P. C. An investigation of the intracellular pH of crab muscle fibres by means of micro-glass and micro-tungsten electrodes. J Physiol. 1954 Oct 28;126(1):169–180. doi: 10.1113/jphysiol.1954.sp005201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiPolo R. Characterization of the ATP-dependent calcium efflux in dialyzed squid giant axons. J Gen Physiol. 1977 Jun;69(6):795–813. doi: 10.1085/jgp.69.6.795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dipolo R., Requena J., Brinley F. J., Jr, Mullins L. J., Scarpa A., Tiffert T. Ionized calcium concentrations in squid axons. J Gen Physiol. 1976 Apr;67(4):433–467. doi: 10.1085/jgp.67.4.433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HINKE J. A. The measurement of sodium and potassium activities in the squid axon by means of cation-selective glass micro-electrodes. J Physiol. 1961 Apr;156:314–335. doi: 10.1113/jphysiol.1961.sp006678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., KEYNES R. D. Movements of labelled calcium in squid giant axons. J Physiol. 1957 Sep 30;138(2):253–281. doi: 10.1113/jphysiol.1957.sp005850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KEYNES R. D., LEWIS P. R. The sodium and potassium content of cephalopod nerve fibers. J Physiol. 1951 Jun;114(1-2):151–182. doi: 10.1113/jphysiol.1951.sp004609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lea T. J., Ashley C. C. Increase in free Ca2+ in muscle after exposure to CO2. Nature. 1978 Sep 21;275(5677):236–238. doi: 10.1038/275236a0. [DOI] [PubMed] [Google Scholar]
- Meech R. W., Thomas R. C. The effect of calcium injection on the intracellular sodium and pH of snail neurones. J Physiol. 1977 Mar;265(3):867–879. doi: 10.1113/jphysiol.1977.sp011749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullins L. J. A mechanism for Na/Ca transport. J Gen Physiol. 1977 Dec;70(6):681–695. doi: 10.1085/jgp.70.6.681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullins L. J., Brinley F. J., Jr Sensitivity of calcium efflux from squid axons to changes in membrane potential. J Gen Physiol. 1975 Feb;65(2):135–152. doi: 10.1085/jgp.65.2.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullins L. J., Brinley F. J., Jr Some factors influencing sodium extrusion by internally dialyzed squid axons. J Gen Physiol. 1967 Nov;50(10):2333–2355. doi: 10.1085/jgp.50.10.2333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullins L. J., Requena J. Calcium measurement in the periphery of an axon. J Gen Physiol. 1979 Sep;74(3):393–413. doi: 10.1085/jgp.74.3.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullins L. J., Requena J. The "late" Ca channel in squid axons. J Gen Physiol. 1981 Dec;78(6):683–700. doi: 10.1085/jgp.78.6.683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Requena J., DiPolo R., Brinley F. J., Jr, Mullins L. J. The control of ionized calcium in squid axons. J Gen Physiol. 1977 Sep;70(3):329–353. doi: 10.1085/jgp.70.3.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Requena J., Mullins L. J., Brinley F. J., Jr Calcium content and net fluxes in squid giant axons. J Gen Physiol. 1979 Mar;73(3):327–342. doi: 10.1085/jgp.73.3.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Requena J., Mullins L. J. Calcium movement in nerve fibres. Q Rev Biophys. 1979 Aug;12(3):371–460. doi: 10.1017/s0033583500005473. [DOI] [PubMed] [Google Scholar]
- Thomas M. V. Arsenazo III forms 2:1 complexes with Ca and 1:1 complexes with Mg under physiological conditions. Estimates of the apparent dissociation constants. Biophys J. 1979 Mar;25(3):541–548. doi: 10.1016/S0006-3495(79)85322-9. [DOI] [PMC free article] [PubMed] [Google Scholar]


