Abstract
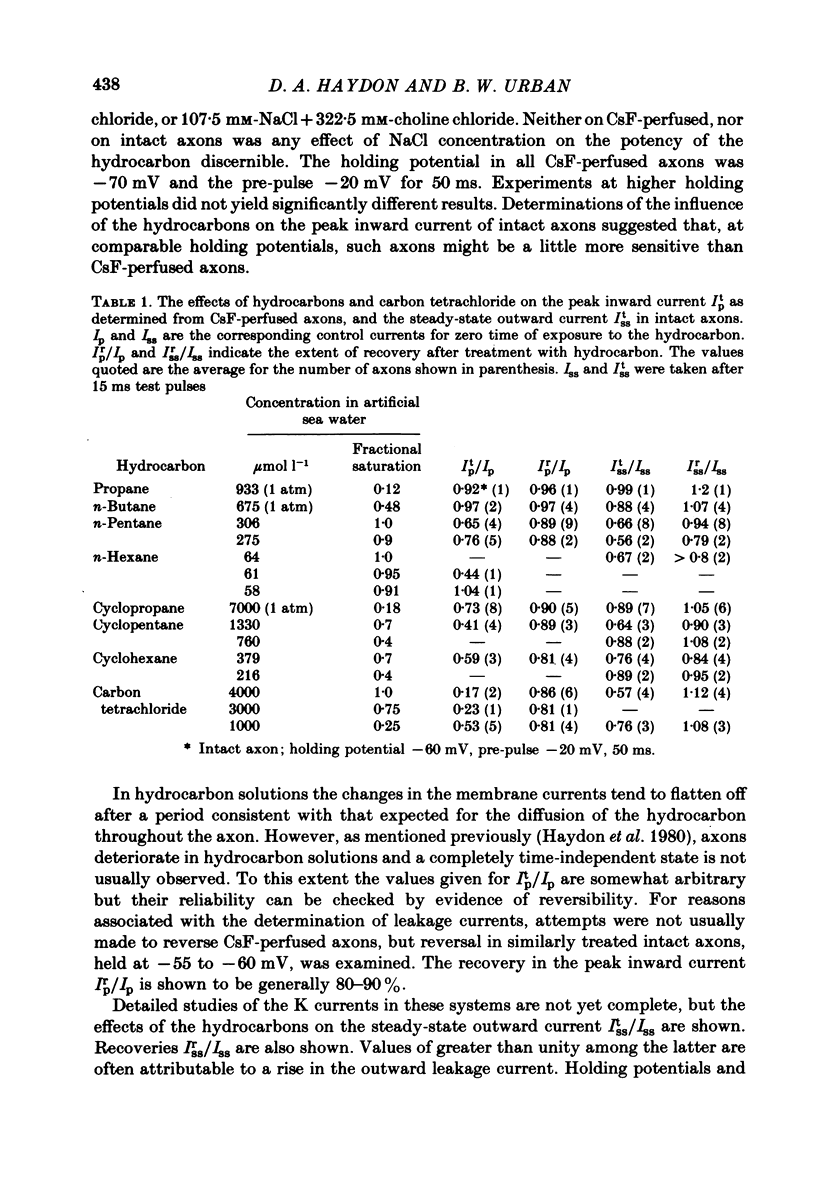
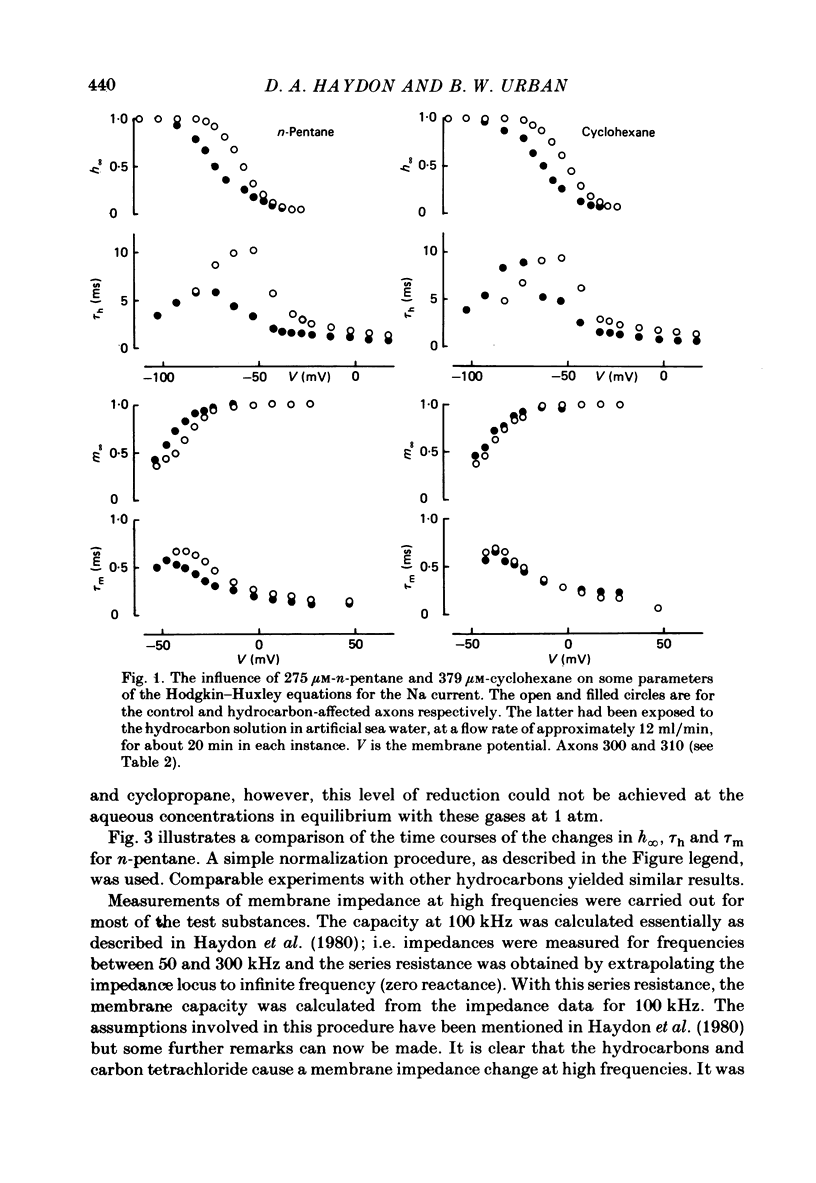
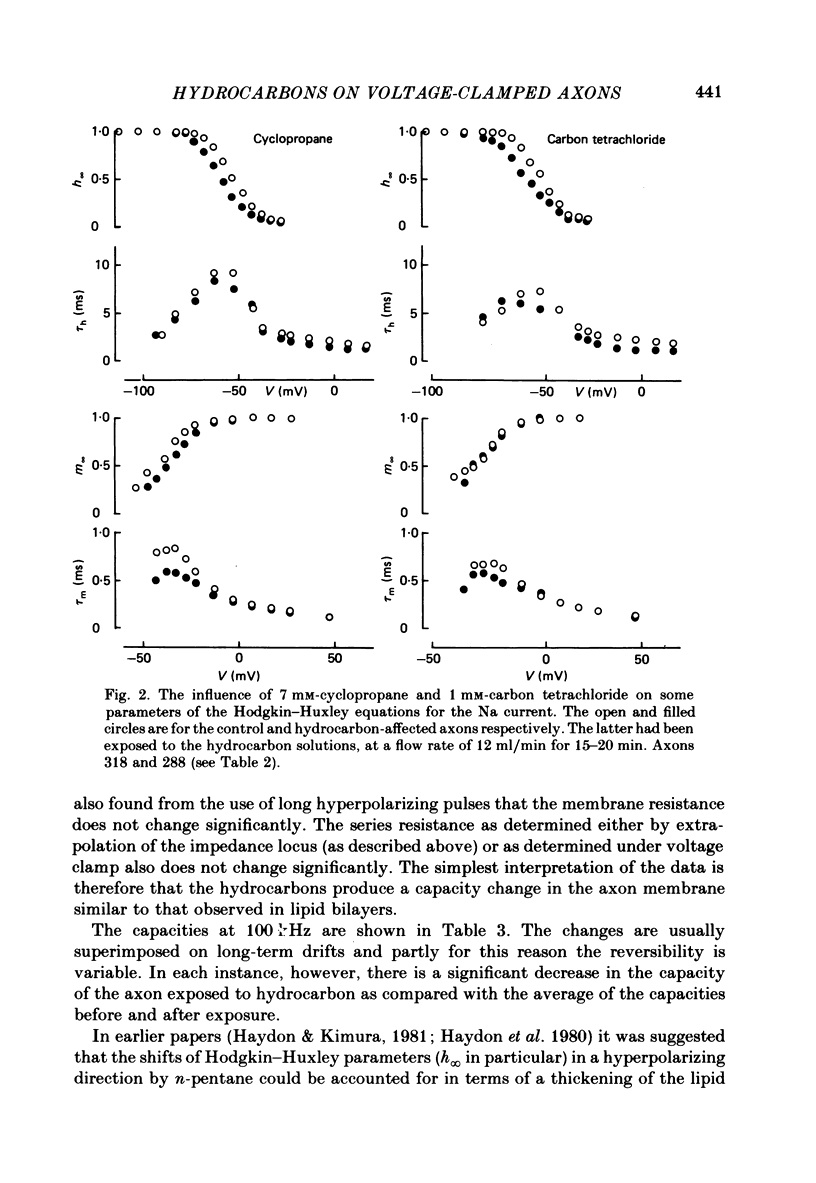
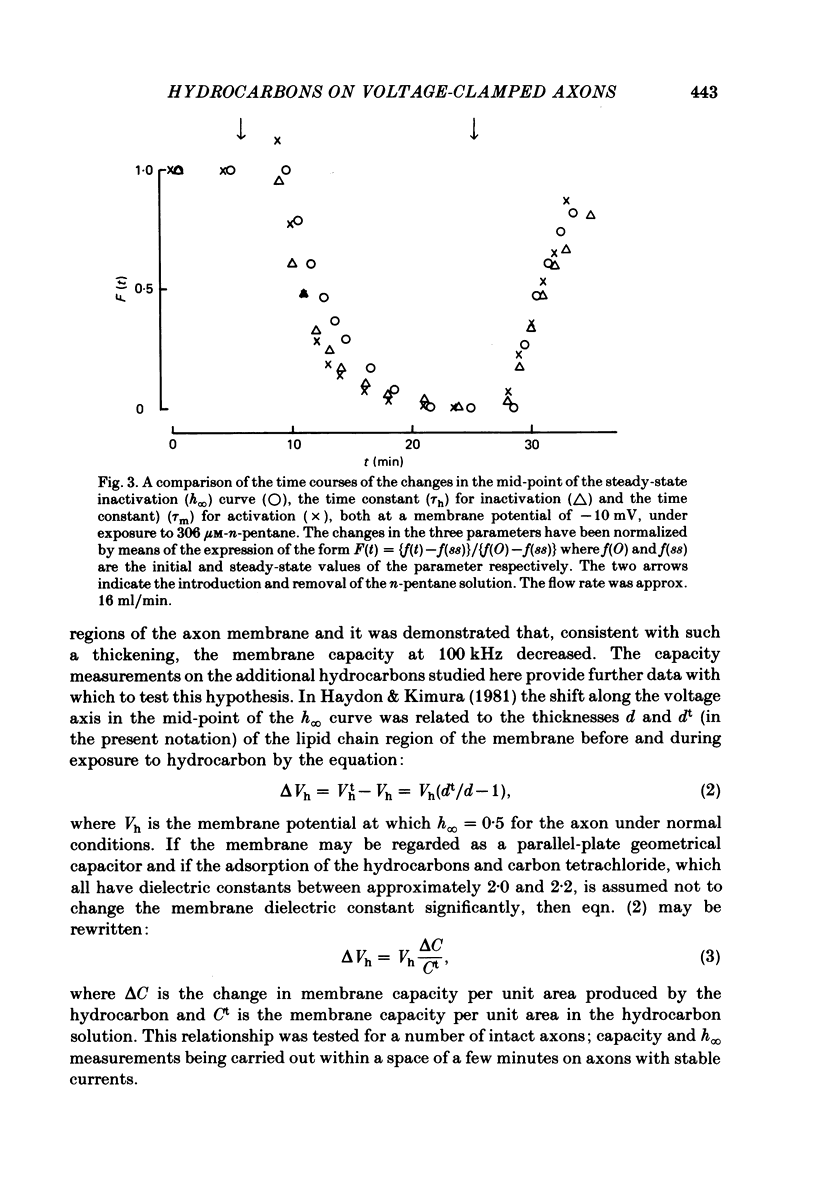
The effects of the n-alkanes propane to hexane, cyclopropane, cyclopentane and cyclohexane and carbon tetrachloride on the ionic currents and electrical capacity of the squid giant axon membrane have been examined. Both the peak inward and steady-state outward currents were reduced reversibly by each substance, though propane at 1 atm had very little effect. The membrane capacity at 100 kHz was reduced by all substances except propane at 1 atm. Na currents were recorded in intracellularly perfused axons before and during exposure to the hydrocarbons and the records were fitted with equations similar to those proposed by Hodgkin & Huxley (1952). Shifts in the curves of the steady-state activation and inactivation parameters (m infinity and h infinity) against membrane potential, changes in the peak heights of the activation and inactivation time constants (tau m and tau h) and reductions in the maximum Na conductance (gNa) have been tabulated. The effects of the various hydrocarbons and carbon tetrachloride on the parameters of the Hodgkin-Huxley equations suggest that the suppression of the Na current by these substances originates from several different phenomena. The underlying physico-chemical events are considered in the light of the observed capacity changes and of information on artificial pore-containing membranes.
Full text
PDF










Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Camejo G., Villegas G. M., Barnola F. V., Villegas R. Characterization of two different membrane fractions isolated from the first stellar nerves of the squid Dosidicus gigas. Biochim Biophys Acta. 1969;193(2):247–259. doi: 10.1016/0005-2736(69)90186-2. [DOI] [PubMed] [Google Scholar]
- Chacko G. K., Villegas G. M., Barnola F. V., Villegas R., Goldman D. E. The polypeptide and the phospholipid components of axon plasma membranes. Biochim Biophys Acta. 1976 Aug 4;443(1):19–32. doi: 10.1016/0005-2736(76)90488-0. [DOI] [PubMed] [Google Scholar]
- Chandler W. K., Hodgkin A. L., Meves H. The effect of changing the internal solution on sodium inactivation and related phenomena in giant axons. J Physiol. 1965 Oct;180(4):821–836. doi: 10.1113/jphysiol.1965.sp007733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franks N. P., Lieb W. R. The structure of lipid bilayers and the effects of general anaesthetics. An x-ray and neutron diffraction study. J Mol Biol. 1979 Oct 9;133(4):469–500. doi: 10.1016/0022-2836(79)90403-0. [DOI] [PubMed] [Google Scholar]
- Gillespie J. I., Meves H. The effect of external potassium on the removal of sodium inactivation in squid giant axons. J Physiol. 1981 Jun;315:493–514. doi: 10.1113/jphysiol.1981.sp013760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruen D. W., Haydon D. A. A mean-field model of the alkane-saturated lipid bilayer above its phase transition. II. Results and comparison with experiment. Biophys J. 1981 Feb;33(2):167–187. doi: 10.1016/S0006-3495(81)84879-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HODGKIN A. L., HUXLEY A. F. A quantitative description of membrane current and its application to conduction and excitation in nerve. J Physiol. 1952 Aug;117(4):500–544. doi: 10.1113/jphysiol.1952.sp004764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haydon D. A. Functions of the lipid in bilayer ion permeability. Ann N Y Acad Sci. 1975 Dec 30;264:2–16. doi: 10.1111/j.1749-6632.1975.tb31472.x. [DOI] [PubMed] [Google Scholar]
- Haydon D. A., Hendry B. M., Levinson S. R., Requena J. Anaesthesia by the n-alkanes. A comparative study of nerve impulse blockage and the properties of black lipid bilayer membranes. Biochim Biophys Acta. 1977 Oct 3;470(1):17–34. doi: 10.1016/0005-2736(77)90058-x. [DOI] [PubMed] [Google Scholar]
- Haydon D. A., Hendry B. M. Nerve impulse blockage in squid axons by n-alkanes: the effect of axon diameter. J Physiol. 1982 Dec;333:393–403. doi: 10.1113/jphysiol.1982.sp014460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haydon D. A., Kimura J. E. Some effects of n-pentane on the sodium and potassium currents of the squid giant axon. J Physiol. 1981 Mar;312:57–70. doi: 10.1113/jphysiol.1981.sp013615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haydon D. A., Requena J., Urban B. W. Some effects of aliphatic hydrocarbons on the electrical capacity and ionic currents of the squid giant axon membrane. J Physiol. 1980 Dec;309:229–245. doi: 10.1113/jphysiol.1980.sp013506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendry B. M., Urban B. W., Haydon D. A. The blockage of the electrical conductance in a pore-containing membrane by the n-alkanes. Biochim Biophys Acta. 1978 Oct 19;513(1):106–116. doi: 10.1016/0005-2736(78)90116-5. [DOI] [PubMed] [Google Scholar]
- Kimura J. E., Meves H. The effect of temperature on the asymmetrical charge movement in squid giant axons. J Physiol. 1979 Apr;289:479–500. doi: 10.1113/jphysiol.1979.sp012748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeman P. The membrane actions of anesthetics and tranquilizers. Pharmacol Rev. 1972 Dec;24(4):583–655. [PubMed] [Google Scholar]


