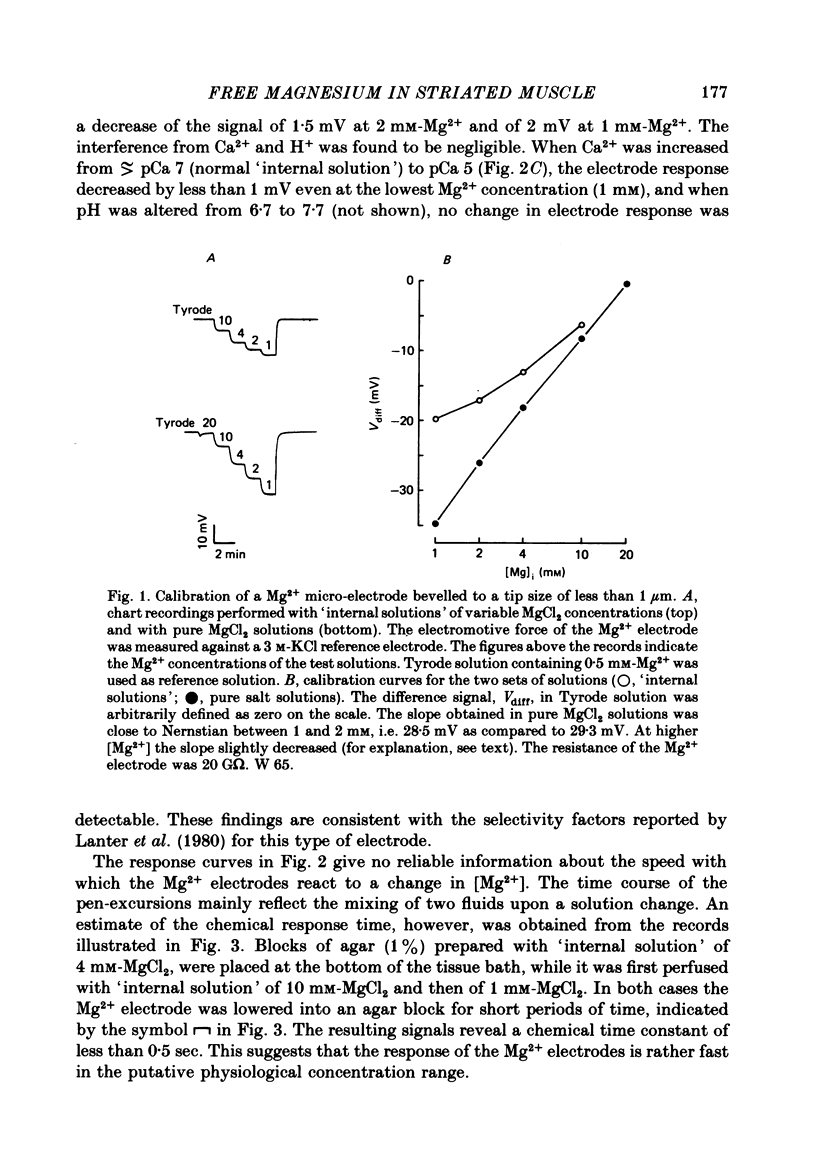
Abstract
1. Neutral carrier-based liquid membrane micro-electrodes were constructed which are suitable for continuous measurements of [Mg2+]i in cardiac and skeletal muscle preparations.
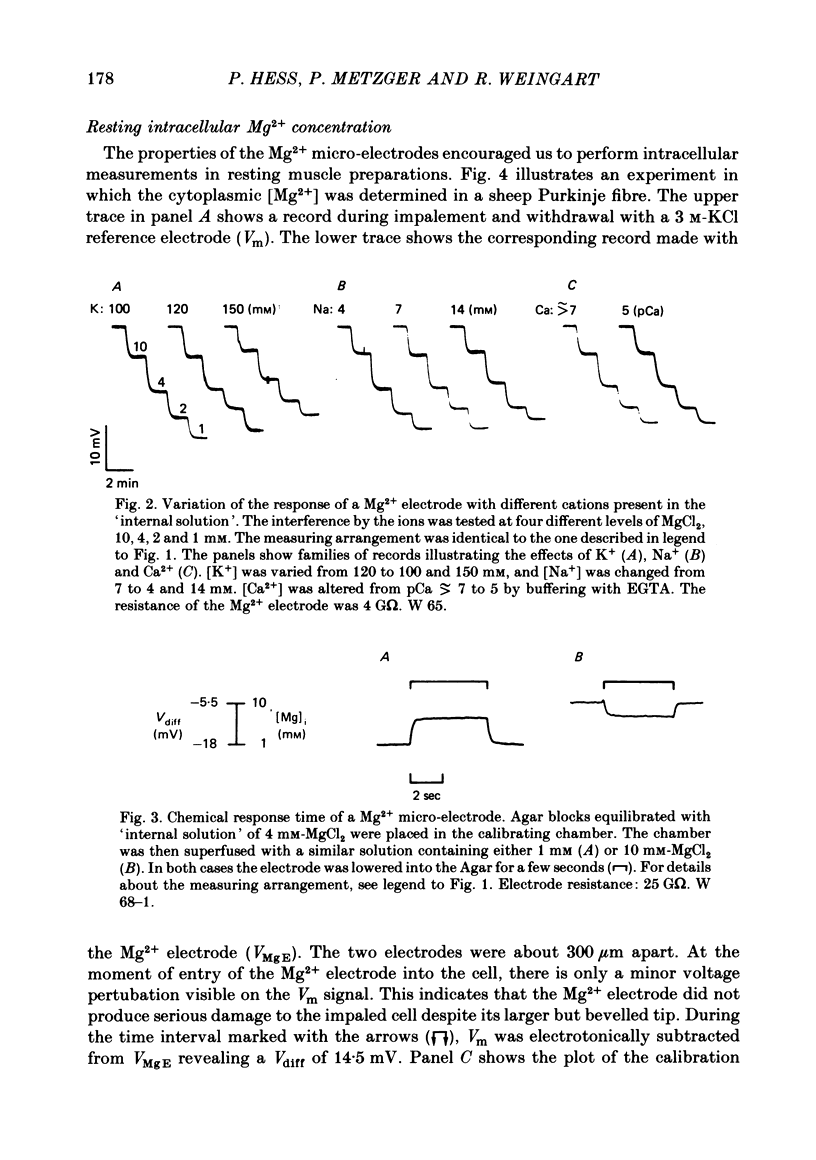
2. The electrodes show a Nernstian behaviour in pure MgCl2 solutions. In the presence of a constant ionic background chosen to simulate the cytoplasmic composition, the calibration function flattens progressively with lower [Mg2+], due to the interference of K+ and Na+. The response to changes in [Mg2+] is less than 0·5 sec.
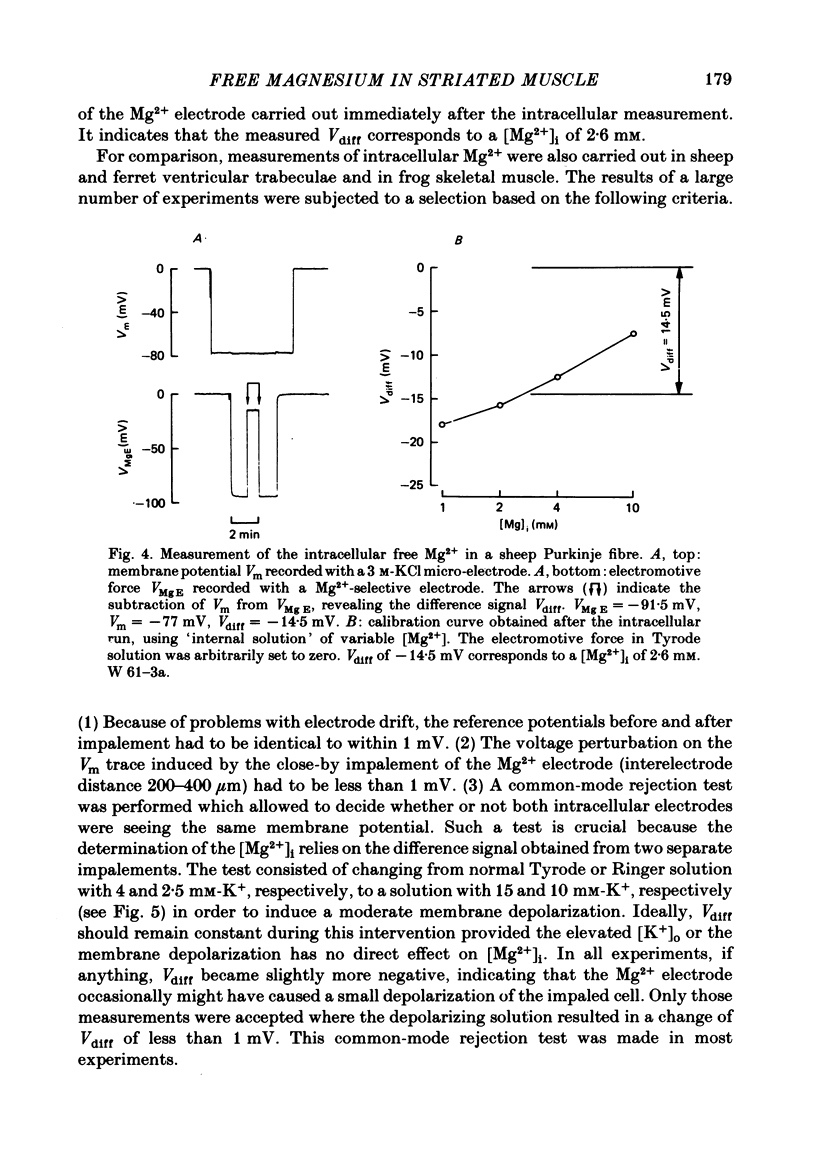
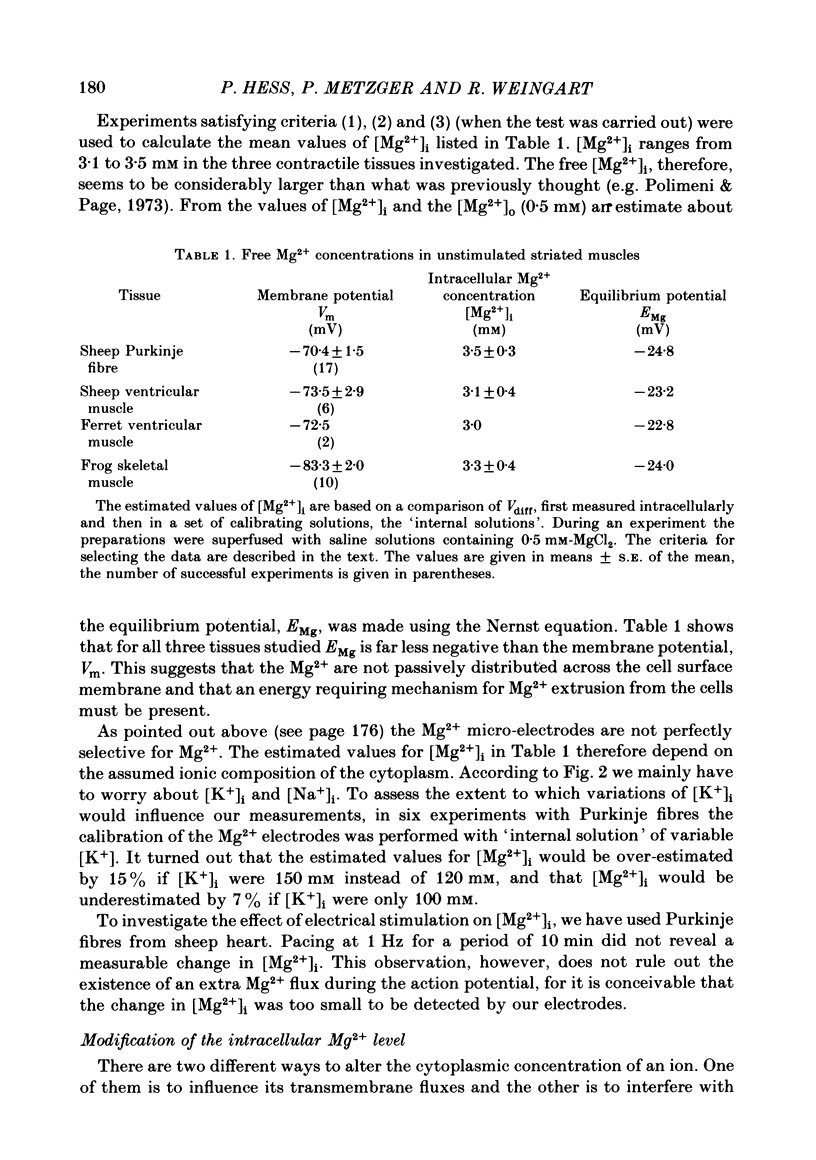
3. In quiescent preparations at room temperature (23 °C), the following basal [Mg2+]i were determined: 3·5 mM (sheep Purkinje fibres), 3·1 mM (sheep ventricular muscle), 3·0 mM (ferret ventricular muscle) and 3·3 mM (frog skeletal muscle).
4. In cardiac tissue, electrical stimulation does not measurably affect the basal [Mg2+]i.
5. In the presence of 0·5 mM-Mg2+o, the calculated Mg2+ equilibrium potentials, EMg, are in the range of -23 to -25 mV, suggesting that Mg2+ is not passively distributed across the sarcolemma in striated muscle.
6. Further studies were performed on sheep Purkinje fibres to investigate the effect of various experimental interventions on [Mg2+]i.
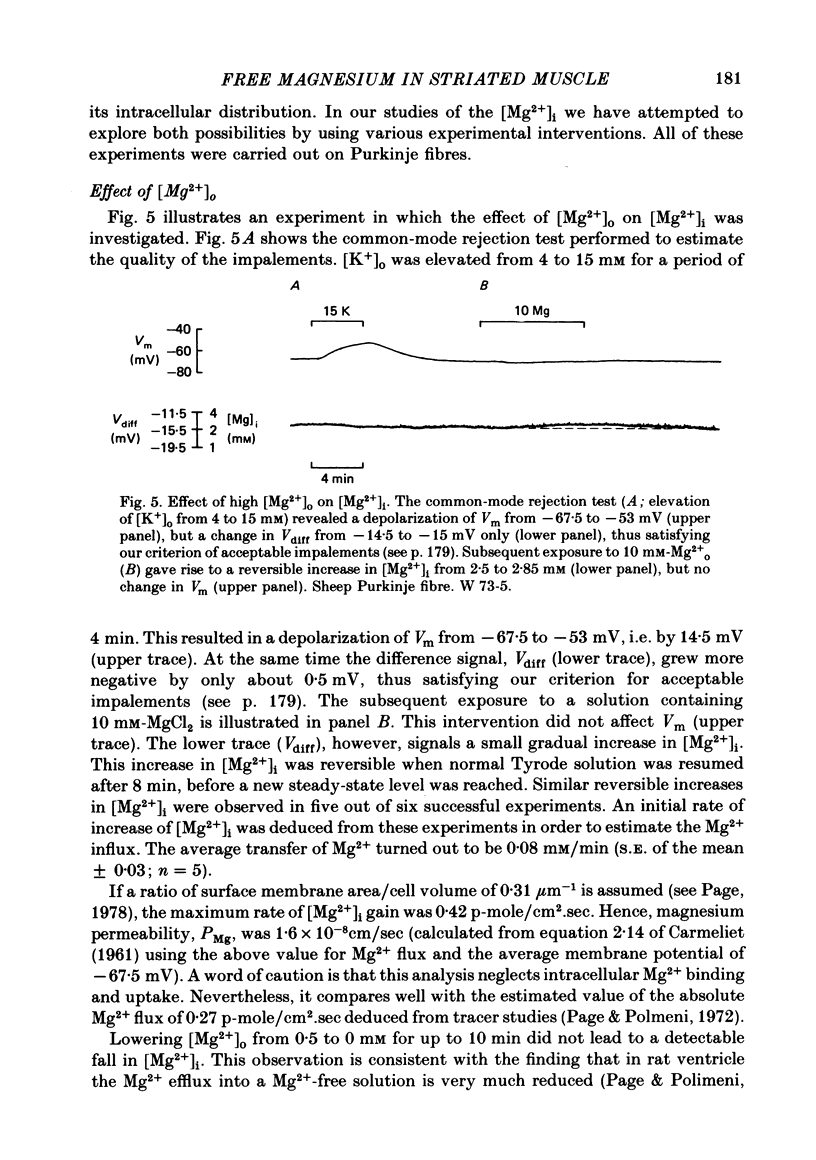
7. Elevating [Mg2+]o from 0·5 to 10 mM resulted in a reversible increase of [Mg2+]i. The initial rate of increase corresponds to a Mg2+ influx of 0·42 p-mole/cm2.sec, or a magnesium permeability, PMg, of 1·6 × 10-8cm/sec.
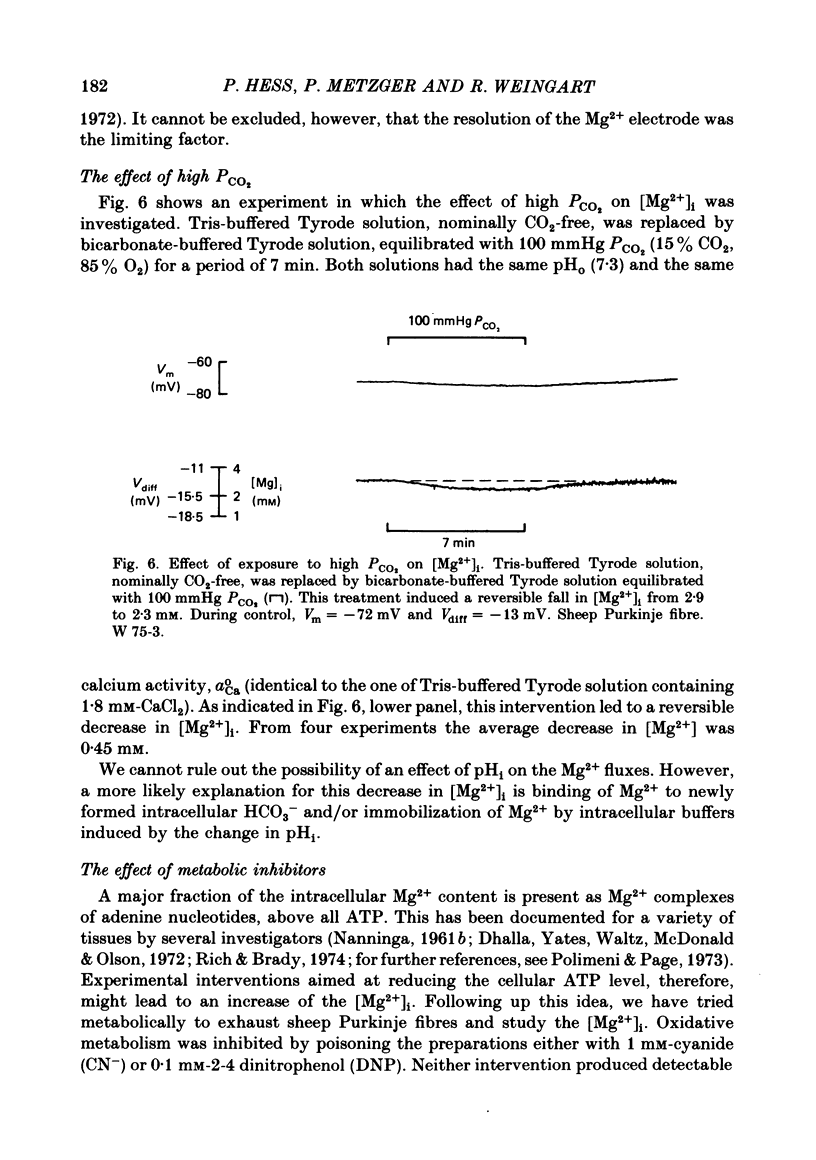
8. Increasing PCO2 from nominally 0 to 100 mmHg (Tris-buffered vs. bicarbonate-buffered Tyrode solution) produced a reversible decrease in [Mg2+]i by roughly 0·45 mM, probably due to Mg2+ binding the newly formed intracellular HCO3-.
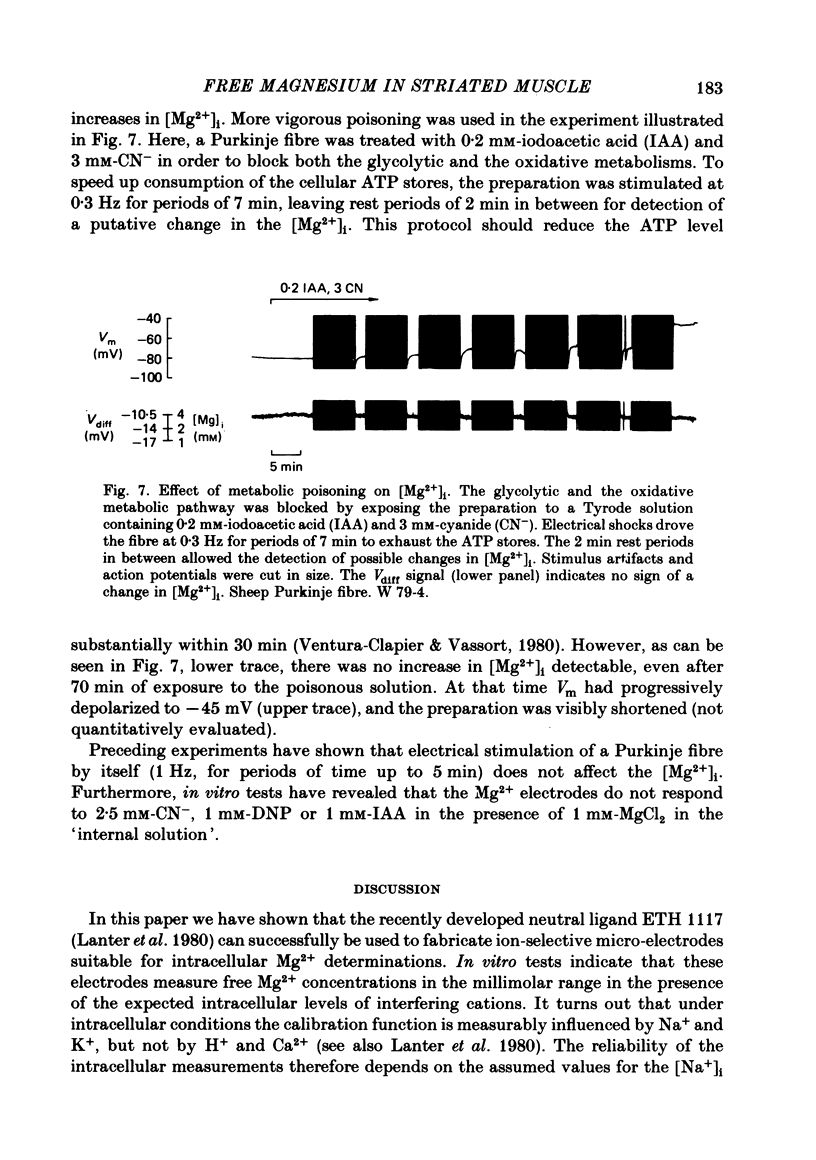
9. The effect of metabolic poisoning on [Mg2+]i was assessed by exposure to cyanide, iodoacetic acid and 2-4-dinitrophenol. No significant increase in [Mg2+]i indicative of a liberation of Mg2+ from ATP was observed.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashley C. C., Ellory J. C. The efflux of magnesium from single crustacean muscle fibres. J Physiol. 1972 Nov;226(3):653–674. doi: 10.1113/jphysiol.1972.sp010002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker P. F., Crawford A. C. Mobility and transport of magnesium in squid giant axons. J Physiol. 1972 Dec;227(3):855–874. doi: 10.1113/jphysiol.1972.sp010062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinley F. J., Jr, Scarpa A., Tiffert T. The concentration of ionized magnesium in barnacle muscle fibres. J Physiol. 1977 Apr;266(3):545–565. doi: 10.1113/jphysiol.1977.sp011781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler J. N. The thermodynamic activity of calcium ion in sodium chloride-calcium chloride electrolytes. Biophys J. 1968 Dec;8(12):1426–1433. doi: 10.1016/S0006-3495(68)86564-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CONWAY E. J. Nature and significance of concentration relations of potassium and sodium ions in skeletal muscle. Physiol Rev. 1957 Jan;37(1):84–132. doi: 10.1152/physrev.1957.37.1.84. [DOI] [PubMed] [Google Scholar]
- Cohen S. M., Burt C. T. 31P nuclear magnetic relaxation studies of phosphocreatine in intact muscle: determination of intracellular free magnesium. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4271–4275. doi: 10.1073/pnas.74.10.4271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhalla N. S., Yates J. C., Walz D. A., McDonald V. A., Olson R. E. Correlation between changes in the endogenous energy stores and myocardial function due to hypoxia in the isolated perfused rat heart. Can J Physiol Pharmacol. 1972 Apr;50(4):333–345. doi: 10.1139/y72-050. [DOI] [PubMed] [Google Scholar]
- Ellis D. The effects of external cations and ouabain on the intracellular sodium activity of sheep heart Purkinje fibres. J Physiol. 1977 Dec;273(1):211–240. doi: 10.1113/jphysiol.1977.sp012090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endo M. Calcium release from the sarcoplasmic reticulum. Physiol Rev. 1977 Jan;57(1):71–108. doi: 10.1152/physrev.1977.57.1.71. [DOI] [PubMed] [Google Scholar]
- Endo M., Iino M. Specific perforation of muscle cell membranes with preserved SR functions by saponin treatment. J Muscle Res Cell Motil. 1980 Mar;1(1):89–100. doi: 10.1007/BF00711927. [DOI] [PubMed] [Google Scholar]
- Endo M., Tanaka M., Ogawa Y. Calcium induced release of calcium from the sarcoplasmic reticulum of skinned skeletal muscle fibres. Nature. 1970 Oct 3;228(5266):34–36. doi: 10.1038/228034a0. [DOI] [PubMed] [Google Scholar]
- Fabiato A., Fabiato F. Calcium release from the sarcoplasmic reticulum. Circ Res. 1977 Feb;40(2):119–129. doi: 10.1161/01.res.40.2.119. [DOI] [PubMed] [Google Scholar]
- Fabiato A., Fabiato F. Contractions induced by a calcium-triggered release of calcium from the sarcoplasmic reticulum of single skinned cardiac cells. J Physiol. 1975 Aug;249(3):469–495. doi: 10.1113/jphysiol.1975.sp011026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabiato A., Fabiato F. Effects of magnesium on contractile activation of skinned cardiac cells. J Physiol. 1975 Aug;249(3):497–517. doi: 10.1113/jphysiol.1975.sp011027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabiato A. Myoplasmic free calcium concentration reached during the twitch of an intact isolated cardiac cell and during calcium-induced release of calcium from the sarcoplasmic reticulum of a skinned cardiac cell from the adult rat or rabbit ventricle. J Gen Physiol. 1981 Nov;78(5):457–497. doi: 10.1085/jgp.78.5.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford L. E., Podolsky R. J. Regenerative calcium release within muscle cells. Science. 1970 Jan 2;167(3914):58–59. doi: 10.1126/science.167.3914.58. [DOI] [PubMed] [Google Scholar]
- GILBERT D. L. Magnesium equilibrium in muscle. J Gen Physiol. 1960 Jul;43:1103–1118. doi: 10.1085/jgp.43.6.1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta R. K., Moore R. D. 31P NMR studies of intracellular free Mg2+ in intact frog skeletal muscle. J Biol Chem. 1980 May 10;255(9):3987–3993. [PubMed] [Google Scholar]
- Kushmerick M. J., Podolsky R. J. Ionic mobility in muscle cells. Science. 1969 Dec 5;166(3910):1297–1298. doi: 10.1126/science.166.3910.1297. [DOI] [PubMed] [Google Scholar]
- Lee C. O., Uhm D. Y., Dresdner K. Sodium-calcium exchange in rabbit heart muscle cells: direct measurement of sarcoplasmic Ca2+ activity. Science. 1980 Aug 8;209(4457):699–701. doi: 10.1126/science.7394527. [DOI] [PubMed] [Google Scholar]
- Loewenstein W. R. Junctional intercellular communication: the cell-to-cell membrane channel. Physiol Rev. 1981 Oct;61(4):829–913. doi: 10.1152/physrev.1981.61.4.829. [DOI] [PubMed] [Google Scholar]
- Marban E., Rink T. J., Tsien R. W., Tsien R. Y. Free calcium in heart muscle at rest and during contraction measured with Ca2+ -sensitive microelectrodes. Nature. 1980 Aug 28;286(5776):845–850. doi: 10.1038/286845a0. [DOI] [PubMed] [Google Scholar]
- Meier P. C., Ammann D., Osswald H. F., Simon W. Review: ion-selective electrodes in clinical chemistry. Med Prog Technol. 1977 Jul 15;5(1):1–12. [PubMed] [Google Scholar]
- NANNINGA L. B. Calculation of free magnesium, calcium and potassium in muscle. Biochim Biophys Acta. 1961 Dec 9;54:338–344. doi: 10.1016/0006-3002(61)90374-2. [DOI] [PubMed] [Google Scholar]
- NANNINGA L. B. The association constant of the complexes of adenosine triphosphate with magnesium, calcium, strontium, and barium ions. Biochim Biophys Acta. 1961 Dec 9;54:330–338. doi: 10.1016/0006-3002(61)90373-0. [DOI] [PubMed] [Google Scholar]
- Page E., Polimeni P. I. Magnesium exchange in rat ventricle. J Physiol. 1972 Jul;224(1):121–139. doi: 10.1113/jphysiol.1972.sp009884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page E. Quantitative ultrastructural analysis in cardiac membrane physiology. Am J Physiol. 1978 Nov;235(5):C147–C158. doi: 10.1152/ajpcell.1978.235.5.C147. [DOI] [PubMed] [Google Scholar]
- Peracchia C., Peracchia L. L. Gap junction dynamics: reversible effects of divalent cations. J Cell Biol. 1980 Dec;87(3 Pt 1):708–718. doi: 10.1083/jcb.87.3.708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polimeni P. I. Extracellular space and ionic distribution in rat ventricle. Am J Physiol. 1974 Sep;227(3):676–683. doi: 10.1152/ajplegacy.1974.227.3.676. [DOI] [PubMed] [Google Scholar]
- Polimeni P. I., Page E. Magnesium in heart muscle. Circ Res. 1973 Oct 5;33(4):367–374. doi: 10.1161/01.res.33.4.367. [DOI] [PubMed] [Google Scholar]
- RAAFLAUB J. Die Metallpufferfunktion der Adenosinphosphate. Helv Physiol Pharmacol Acta. 1956;14(3):304–318. [PubMed] [Google Scholar]
- Rich T. L., Brady A. J. Potassium contracture and utilization of high-energy phosphates in rabbit heart. Am J Physiol. 1974 Jan;226(1):105–113. doi: 10.1152/ajplegacy.1974.226.1.105. [DOI] [PubMed] [Google Scholar]
- Scarpa A., Graziotti P. Mechanisms for intracellular calcium regulation in heart. I. Stopped-flow measurements of Ca++ uptake by cardiac mitochondria. J Gen Physiol. 1973 Dec;62(6):756–772. doi: 10.1085/jgp.62.6.756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shatkay A. Individual activity of calcium ions in pure solutions of CaCl2 and in mixtures. Biophys J. 1968 Aug;8(8):912–919. doi: 10.1016/S0006-3495(68)86528-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shine K. I. Myocardial effects of magnesium. Am J Physiol. 1979 Oct;237(4):H413–H423. doi: 10.1152/ajpheart.1979.237.4.H413. [DOI] [PubMed] [Google Scholar]
- Stephenson E. W., Podolsky R. J. Regulation by magnesium of intracellular calcium movement in skinned muscle fibers. J Gen Physiol. 1977 Jan;69(1):1–16. doi: 10.1085/jgp.69.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsien R. Y., Rink T. J. Neutral carrier ion-selective microelectrodes for measurement of intracellular free calcium. Biochim Biophys Acta. 1980 Jul;599(2):623–638. doi: 10.1016/0005-2736(80)90205-9. [DOI] [PubMed] [Google Scholar]
- Walker J. L., Brown H. M. Intracellular ionic activity measurements in nerve and muscle. Physiol Rev. 1977 Oct;57(4):729–778. doi: 10.1152/physrev.1977.57.4.729. [DOI] [PubMed] [Google Scholar]