Abstract
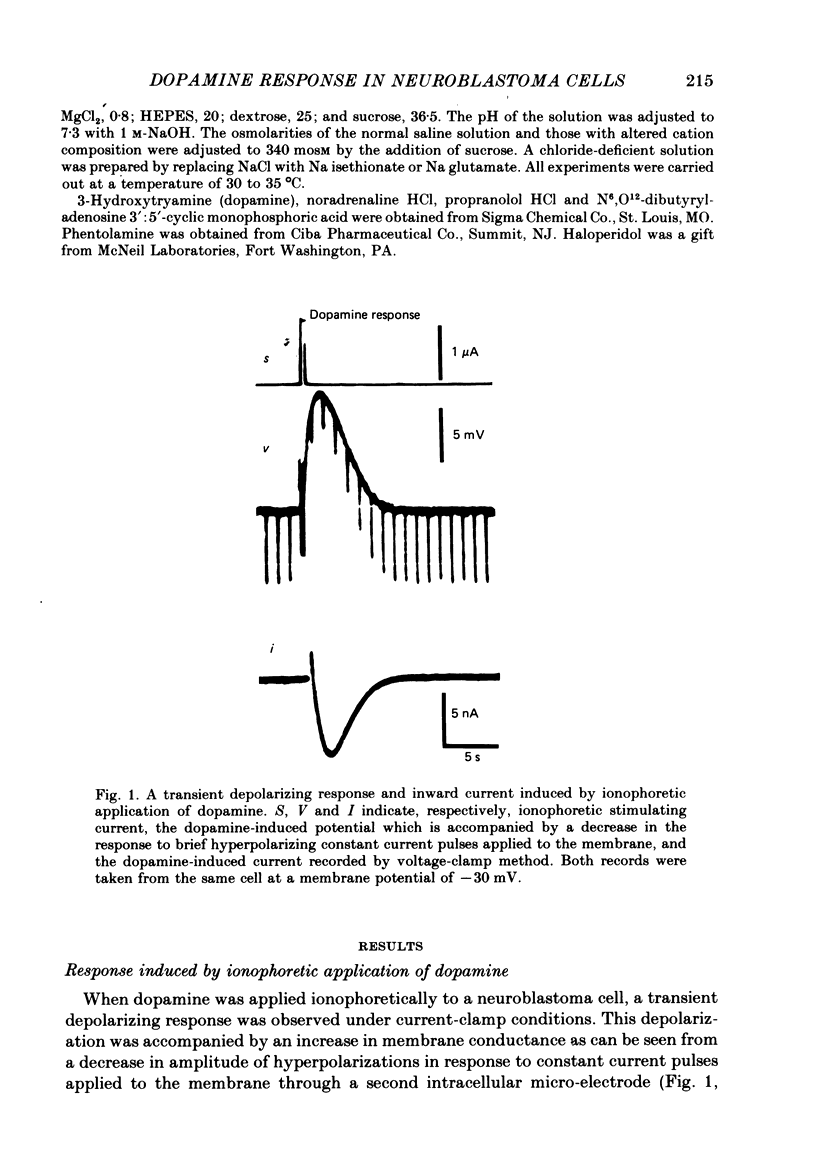
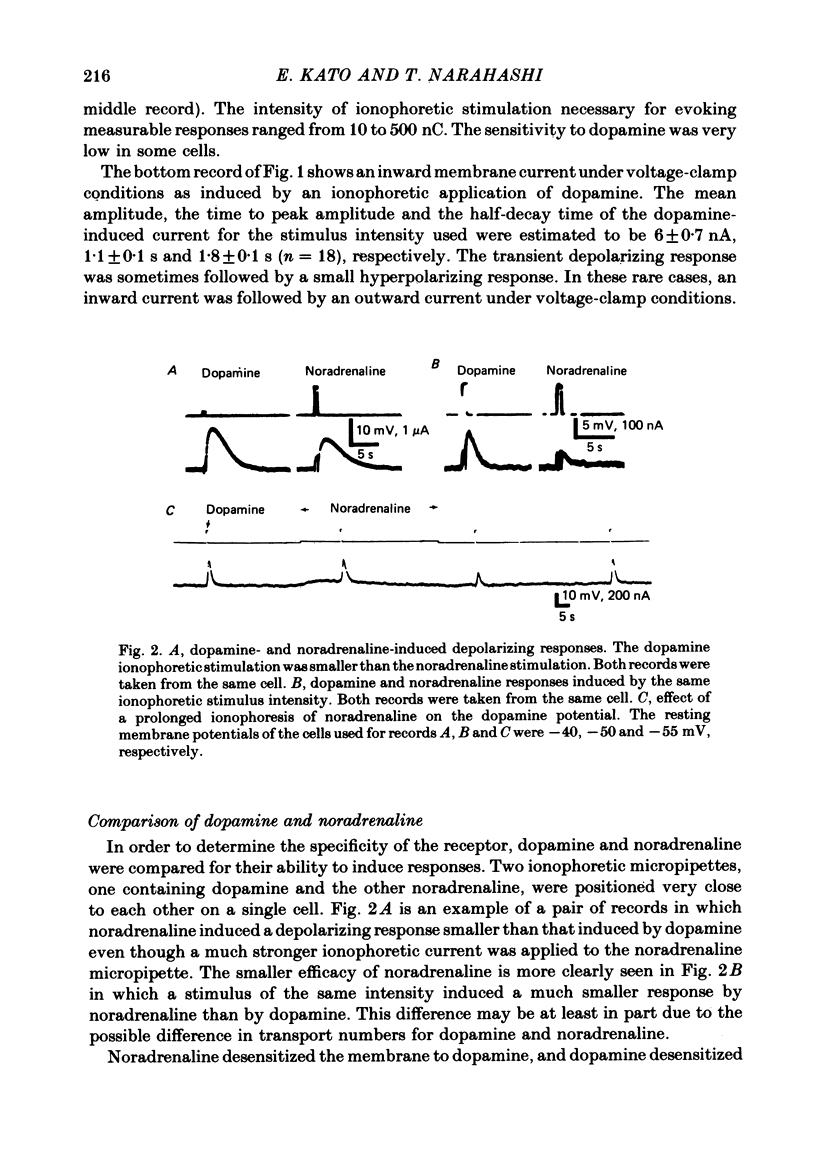
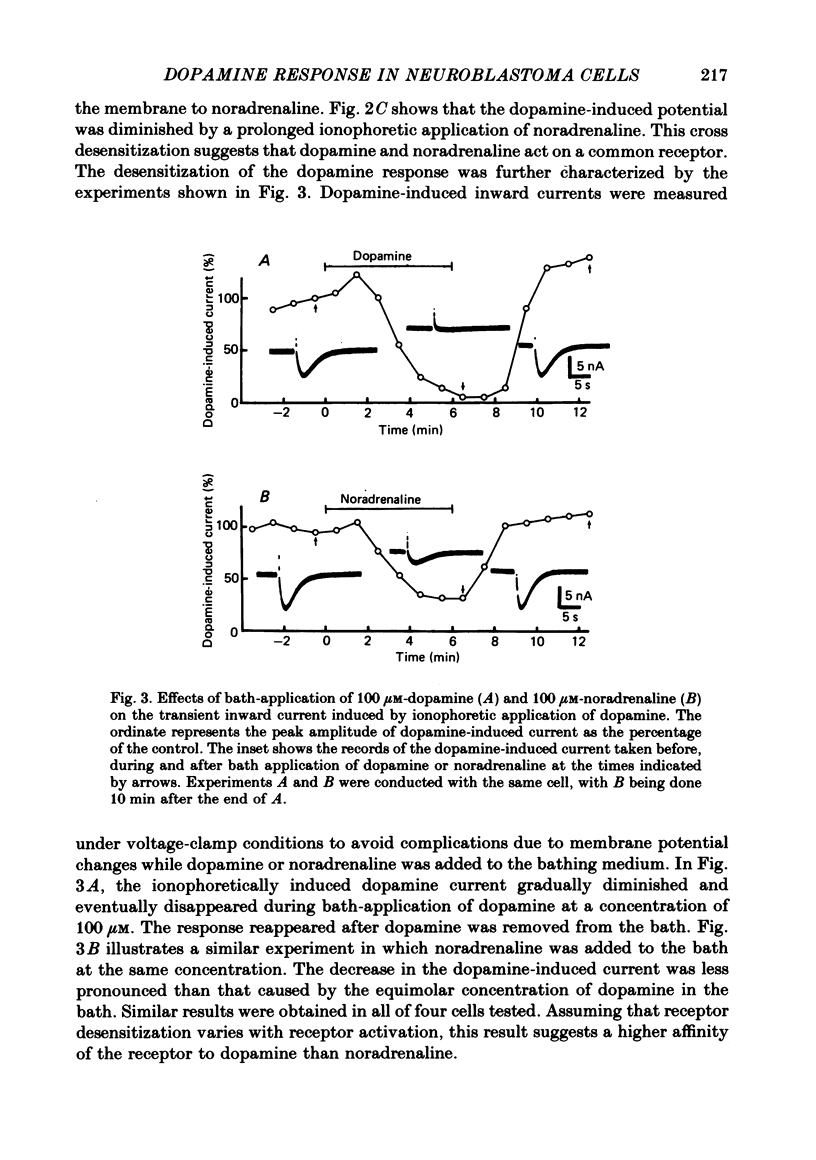
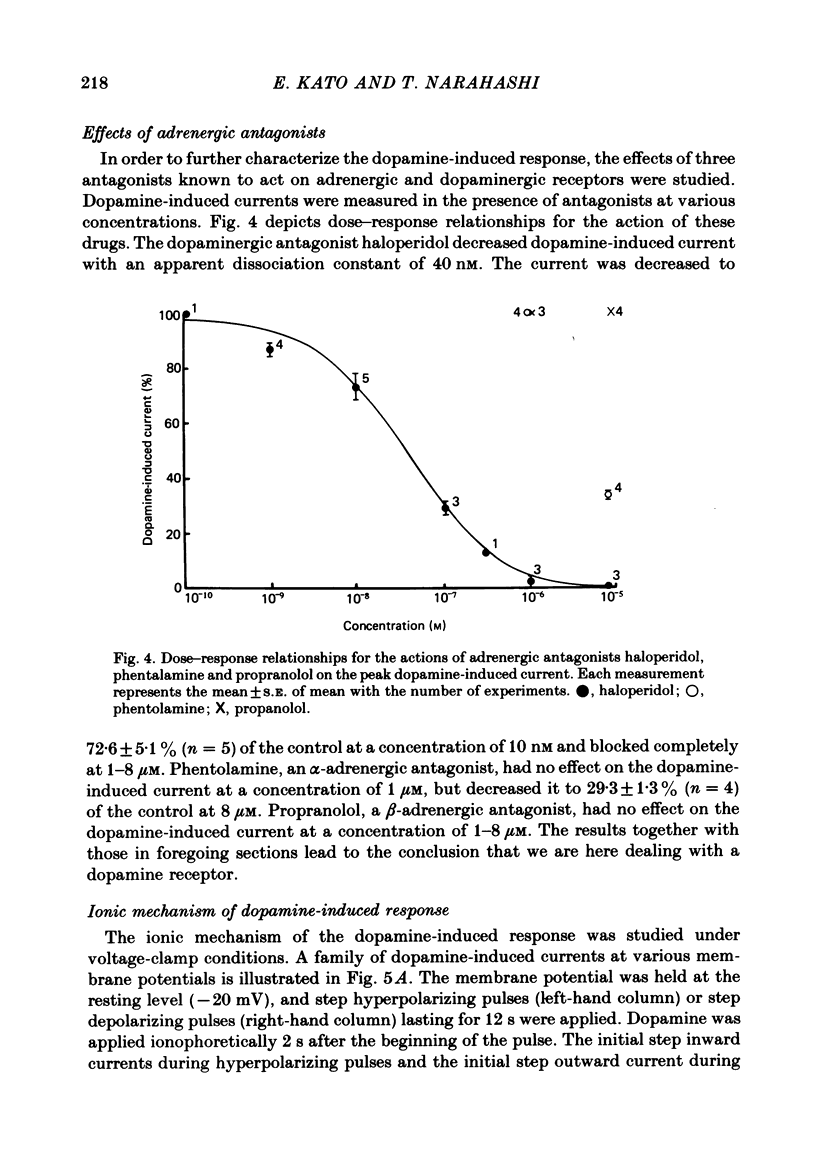
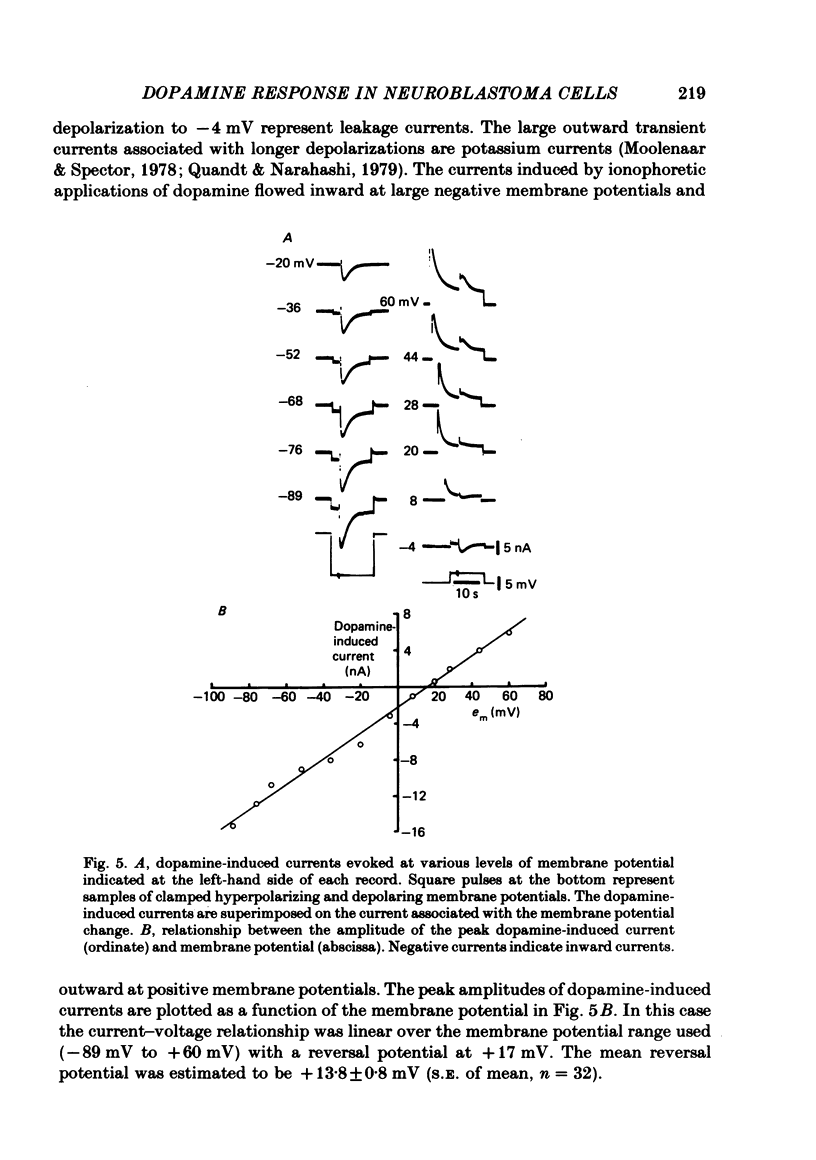
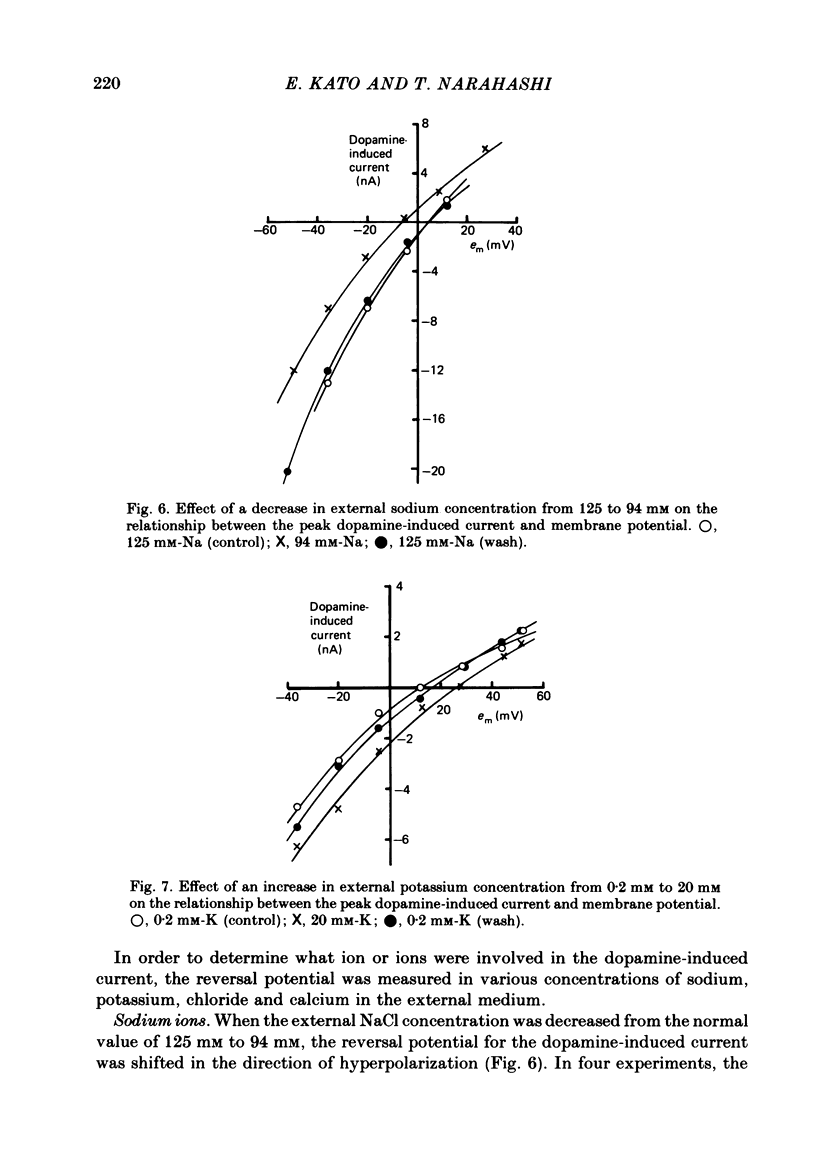
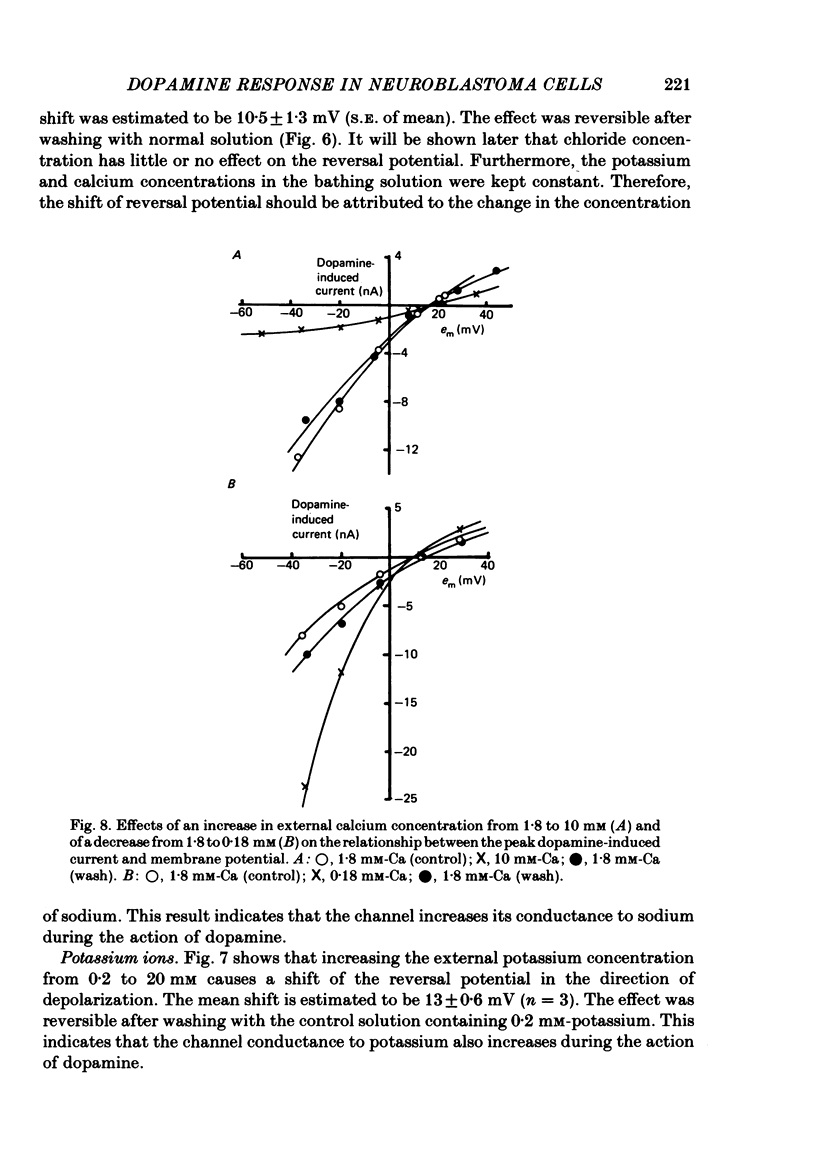
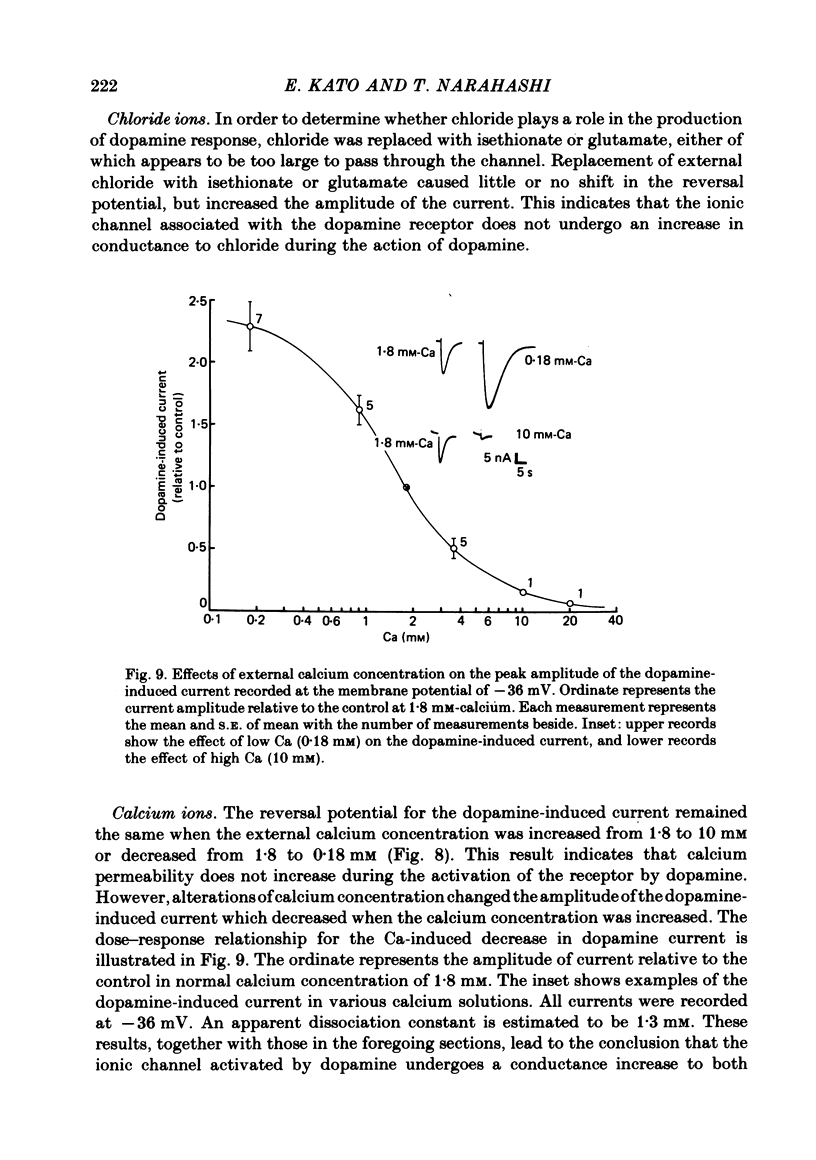
1. The characteristics of the electrical response to dopamine in the mouse neuroblastoma cell line N1E-115 were studied. 2. Neuroblastoma cells responded to ionophoretically applied dopamine by generating a transient depolarization. Under voltage-clamp conditions, a transient inward current was recorded in response to dopamine application. 3. The receptor was more effectively activated by dopamine than by noradrenaline. Haloperidol blocked the dopamine-induced current with an apparent dissociation constant of 40 nM. Phentolamine was much less potent than haloperidol, and propranolol had no effect. 4. The dopamine-induced current was increased in amplitude by hyperpolarizing the membrane, decreased by depolarization, and reversed its polarity at + 14 mV. 5. When the external sodium concentration was decreased from 125 to 94 mM, the reversal potential was shifted in the direction of hyperpolarization by 10 mV. 6. Increasing the external potassium concentration from 0.2 to 20 mM caused a shift of the reversal potential by 13 mV in the direction of depolarization. 7. Replacement of external chloride with isethionate or glutamate caused little or no shift in the reversal potential, but increased the amplitude of the current. 8. Increase in external calcium concentration caused a block of the dopamine-induced current with an apparent dissociation constant of 1.3 mM, without altering its reversal potential. 9. It is concluded that the ionic channel activated by dopamine undergoes a conductance increase to both sodium and potassium but not to chloride or calcium.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amano T., Richelson E., Nirenberg M. Neurotransmitter synthesis by neuroblastoma clones (neuroblast differentiation-cell culture-choline acetyltransferase-acetylcholinesterase-tyrosine hydroxylase-axons-dendrites). Proc Natl Acad Sci U S A. 1972 Jan;69(1):258–263. doi: 10.1073/pnas.69.1.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breakefield X. O. Reserpine sensitivity of catecholamine metabolism in murine neuroblastoma clone N1E-115. J Neurochem. 1975 Dec;25(6):877–882. doi: 10.1111/j.1471-4159.1975.tb04421.x. [DOI] [PubMed] [Google Scholar]
- Christian C. N., Nelson P. G., Bullock P., Mullinax D., Nirenberg M. Pharmacologic responses of cells of a neuroblastoma X glioma hybrid clone and modulation of synapses between hybrid cells and mouse myotubes. Brain Res. 1978 May 26;147(2):261–276. doi: 10.1016/0006-8993(78)90839-9. [DOI] [PubMed] [Google Scholar]
- Commissiong J. W., Neff N. H. Current status of dopamine in the mammalian spinal cord. Biochem Pharmacol. 1979 May 15;28(10):1569–1573. doi: 10.1016/0006-2952(79)90166-7. [DOI] [PubMed] [Google Scholar]
- Creese I., Sibley D. R., Leff S., Hamblin M. Dopamine receptors: subtypes, localization and regulation. Fed Proc. 1981 Feb;40(2):147–152. [PubMed] [Google Scholar]
- De Potter W. P., Fraeyman N. H., Palm J. W., De Schaepdryver A. F. Localization of noradrenaline and dopamine-beta-hydroxylase in C1300 mouse neuroblastoma. A biochemical and electronmicroscopic study. Life Sci. 1978 Dec 31;23(27-28):2665–2674. doi: 10.1016/0024-3205(78)90645-8. [DOI] [PubMed] [Google Scholar]
- Deguchi T., Narahashi T. Effects of procaine on ionic conductances of end-plate membranes. J Pharmacol Exp Ther. 1971 Feb;176(2):423–433. [PubMed] [Google Scholar]
- Donnelly C. H., Richelson E., Murphy D. L. Properties of monoamine oxidase in mouse neuroblastoma N1E-115 cells. Biochem Pharmacol. 1976 Jul 15;25(14):1639–1643. doi: 10.1016/0006-2952(76)90476-7. [DOI] [PubMed] [Google Scholar]
- Hawkins M., Jr, Breakefield X. O. Monoamine oxidase A and B in cultured cells. J Neurochem. 1978 Jun;30(6):1391–1397. doi: 10.1111/j.1471-4159.1978.tb10471.x. [DOI] [PubMed] [Google Scholar]
- Kanno M., Dunning B. B., Machne X. Calcium dependence of acetylcholine induced conductance changes. Life Sci. 1976 Feb 1;18(3):311–318. doi: 10.1016/0024-3205(76)90059-x. [DOI] [PubMed] [Google Scholar]
- Koketsu K. Cholinergic synaptic potentials and the underlying ionic mechasims. Fed Proc. 1969 Jan-Feb;28(1):101–112. [PubMed] [Google Scholar]
- Kuba K., Nishi S. Characteristics of fast excitatory postsynaptic current in bullfrog sympathetic ganglion cells. Effects of membrane potential, temperature and Ca ions. Pflugers Arch. 1979 Jan 31;378(3):205–212. doi: 10.1007/BF00592737. [DOI] [PubMed] [Google Scholar]
- MacDermott A. B., Connor E. A., Dionne V. E., Parsons R. L. Voltage clamp study of fast excitatory synaptic currents in bullfrog sympathetic ganglion cells. J Gen Physiol. 1980 Jan;75(1):39–60. doi: 10.1085/jgp.75.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moolenaar W. H., Spector I. Ionic currents in cultured mouse neuroblastoma cells under voltage-clamp conditions. J Physiol. 1978 May;278:265–286. doi: 10.1113/jphysiol.1978.sp012303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers P. R., Livengood D. R. Dopamine depolarising response in a vertebrate neuronal somatic cell hybrid. Nature. 1975 May 15;255(5505):235–236. doi: 10.1038/255235a0. [DOI] [PubMed] [Google Scholar]
- Myers P. R., Livengood D. R., Shain W. Characterization of a depolarizing dopamine response in a vertebrate neuronal somatic cell hybrid. J Cell Physiol. 1977 Apr;91(1):103–118. doi: 10.1002/jcp.1040910111. [DOI] [PubMed] [Google Scholar]
- Narotzky R., Bondareff W. Biogenic amines in cultured neuroblastoma and astrocytoma cells. J Cell Biol. 1974 Oct;63(1):64–70. doi: 10.1083/jcb.63.1.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peacock J. H., Nelson P. G. Chemosensitivity of mouse neuroblastoma cells in vitro. J Neurobiol. 1973;4(4):363–374. doi: 10.1002/neu.480040405. [DOI] [PubMed] [Google Scholar]
- Richelson E. Properties of tyrosine hydroxylation in living mouse neuroblastoma clone N1E-115. J Neurochem. 1976 Nov;27(5):1113–1118. doi: 10.1111/j.1471-4159.1976.tb00317.x. [DOI] [PubMed] [Google Scholar]
- Richelson E. Stimulation of tyrosine hydroxylase activity in an adrenergic clone of mouse neuroblastoma by dibutyryl cyclic AMP. Nat New Biol. 1973 Apr 11;242(119):175–177. doi: 10.1038/newbio242175a0. [DOI] [PubMed] [Google Scholar]
- Richelson E. Studies on the transport of L-tyrosine into an adrenergic clone of mouse neuroblastoma. J Biol Chem. 1974 Oct 10;249(19):6218–6224. [PubMed] [Google Scholar]
- Richelson E., Thompson E. J. Transport of neurotransmitter precursors into cultured cells. Nat New Biol. 1973 Feb 14;241(111):201–204. doi: 10.1038/newbio241201a0. [DOI] [PubMed] [Google Scholar]
- Skaper S. D., Adelson G. L., Seegmiller J. E. Metabolism of biogenic amines in neuroblastoma and glioma cells in culture. J Neurochem. 1976 Nov;27(5):1065–1070. doi: 10.1111/j.1471-4159.1976.tb00309.x. [DOI] [PubMed] [Google Scholar]
- TAKEUCHI A., TAKEUCHI N. On the permeability of end-plate membrane during the action of transmitter. J Physiol. 1960 Nov;154:52–67. doi: 10.1113/jphysiol.1960.sp006564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TAKEUCHI N. Effects of calcium on the conductance change of the end-plate membrane during the action of transmitter. J Physiol. 1963 Jun;167:141–155. doi: 10.1113/jphysiol.1963.sp007137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traber J., Reiser G., Fischer K., Hamprecht B. Measurements of adenosine 3':5'-cyclic monophosphate and membrane potential in neuroblastoma times glioma hybrid cells: opiates and adrenergic agonists cause effects opposite to those of prostaglandin E1. FEBS Lett. 1975 Apr 1;52(2):327–332. doi: 10.1016/0014-5793(75)80836-2. [DOI] [PubMed] [Google Scholar]
- Waymire J. C., Weiner N., Prasad K. N. Regulation of tyrosine hydroxylase activity in cultured mouse neuroblastoma cells: elevation induced by analogs of adenosine 3':5'-cyclic monophosphate. Proc Natl Acad Sci U S A. 1972 Aug;69(8):2241–2245. doi: 10.1073/pnas.69.8.2241. [DOI] [PMC free article] [PubMed] [Google Scholar]


