Abstract
1. Simultaneous measurements of voltage-clamp currents and tension were made in shortened sheep Purkinje fibres exposed to various concentrations of strophanthidin, ouabain and digoxin.
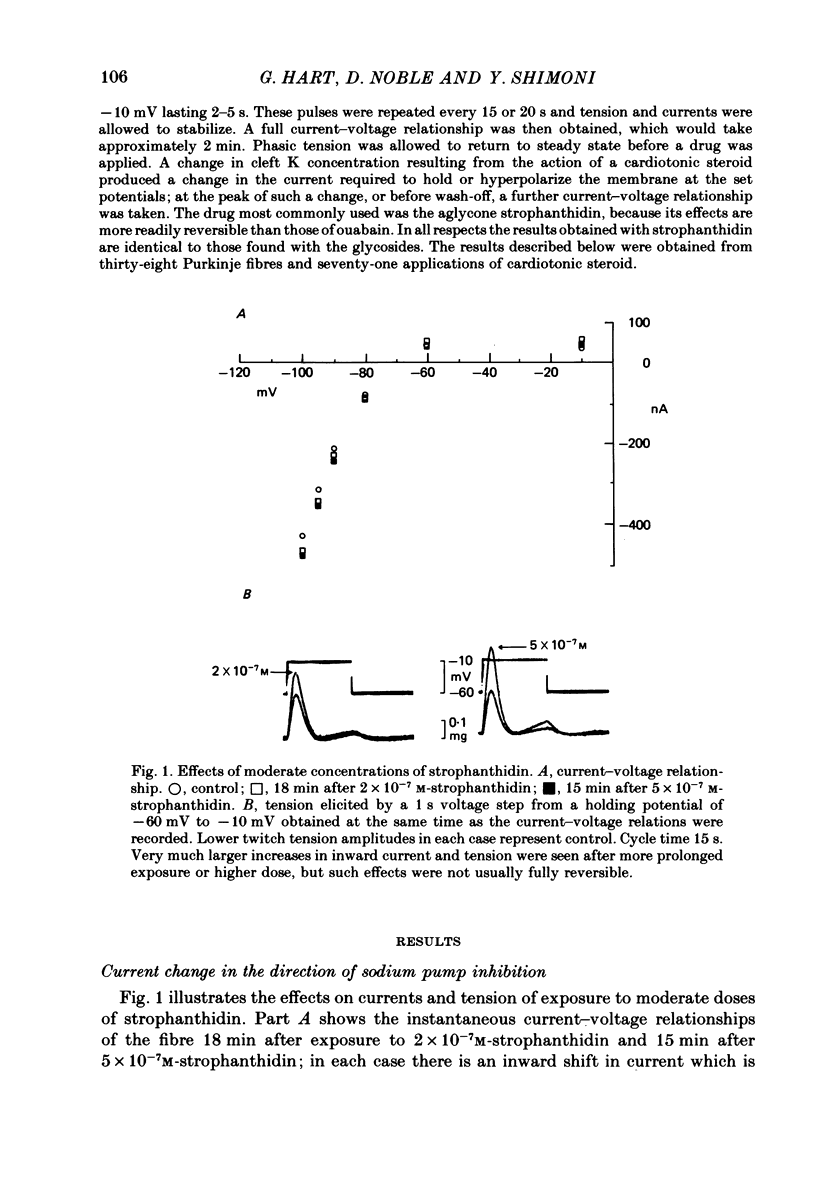
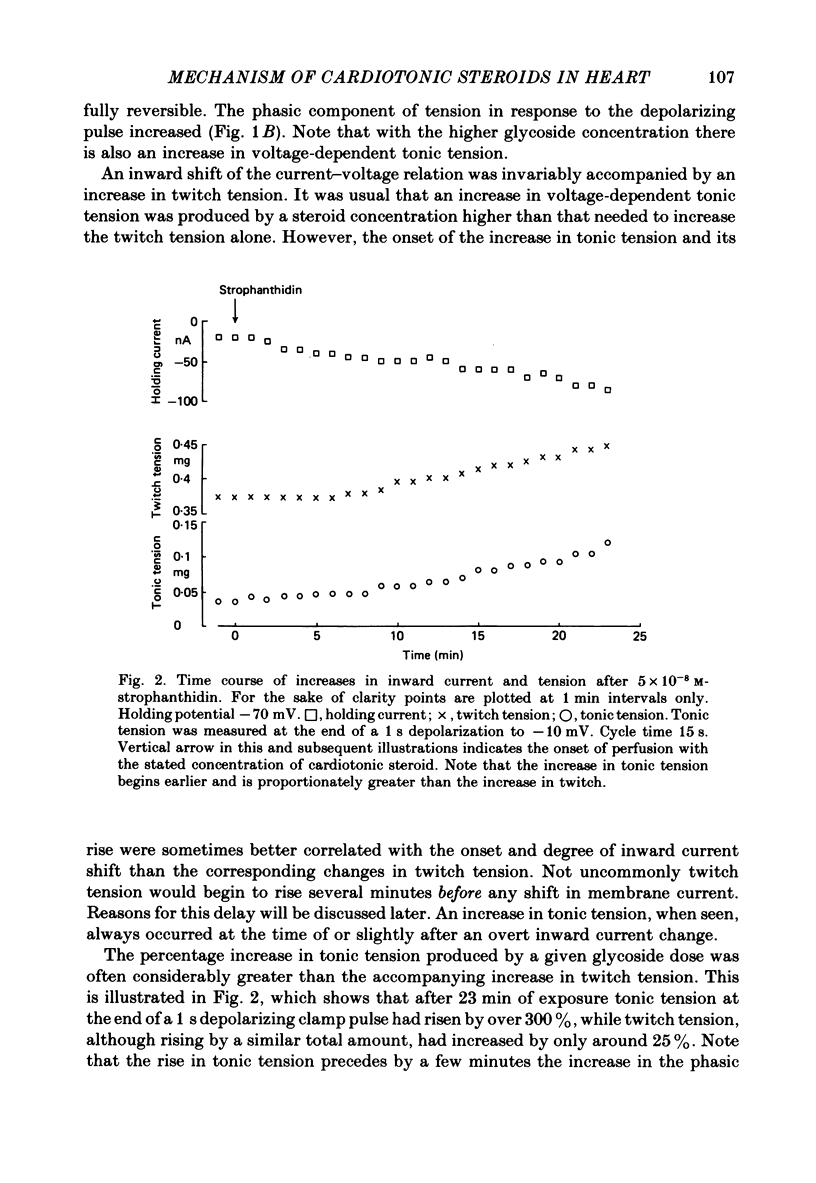
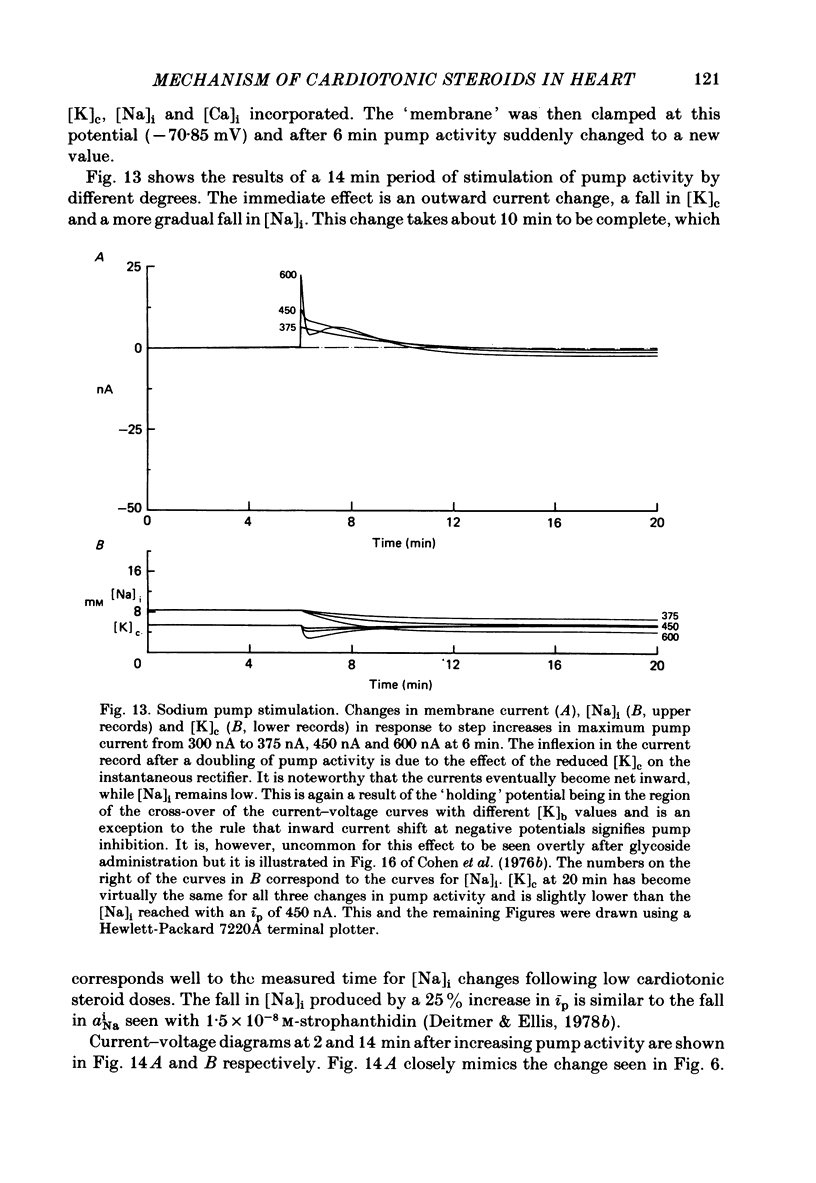
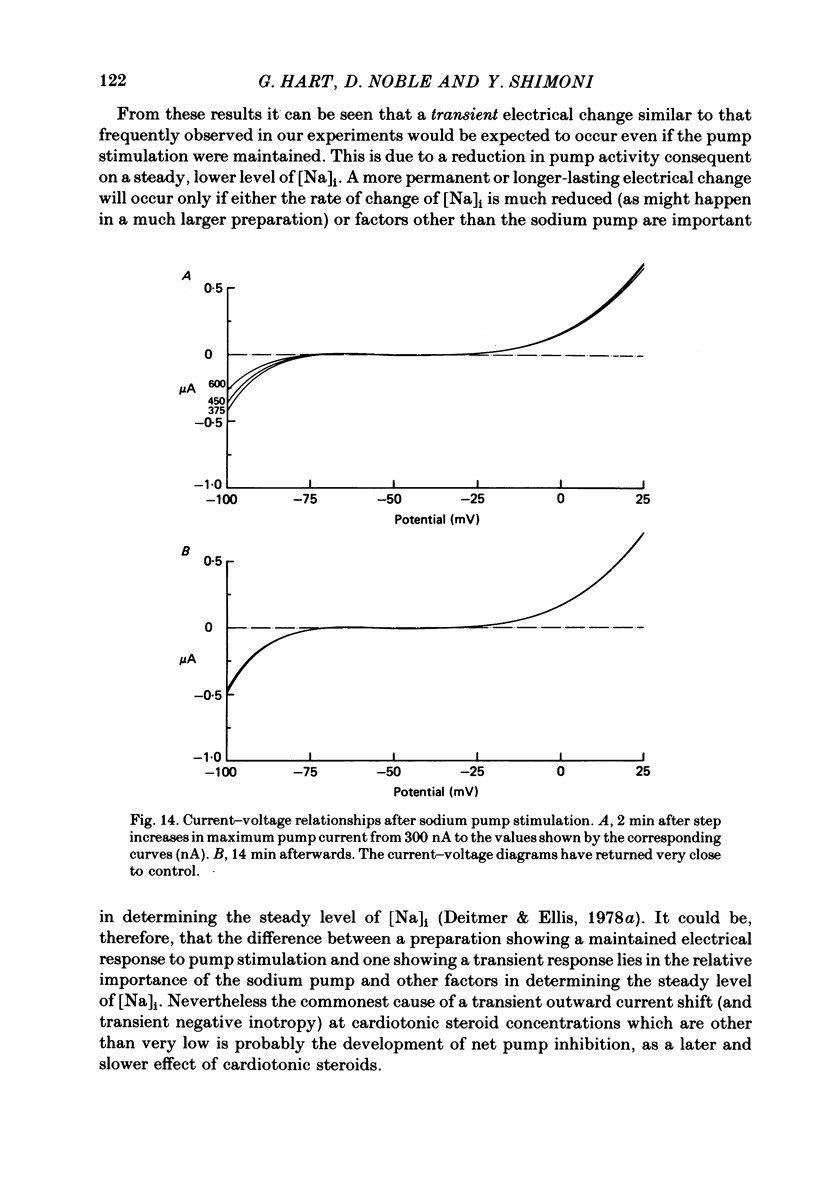
2. In 5·4 mM-K moderate doses (mean 2·4 × 10-7M) of the drugs produced an inward shift of the current—voltage relationship at very negative potentials, consistent with an increase in cleft K concentration (Cohen, Daut & Noble, 1976b), which was always accompanied by an increase in tension. This change, which has been attributed to Na—K pump inhibition, was often better correlated with an increase in voltage-dependent tonic tension than in twitch tension.
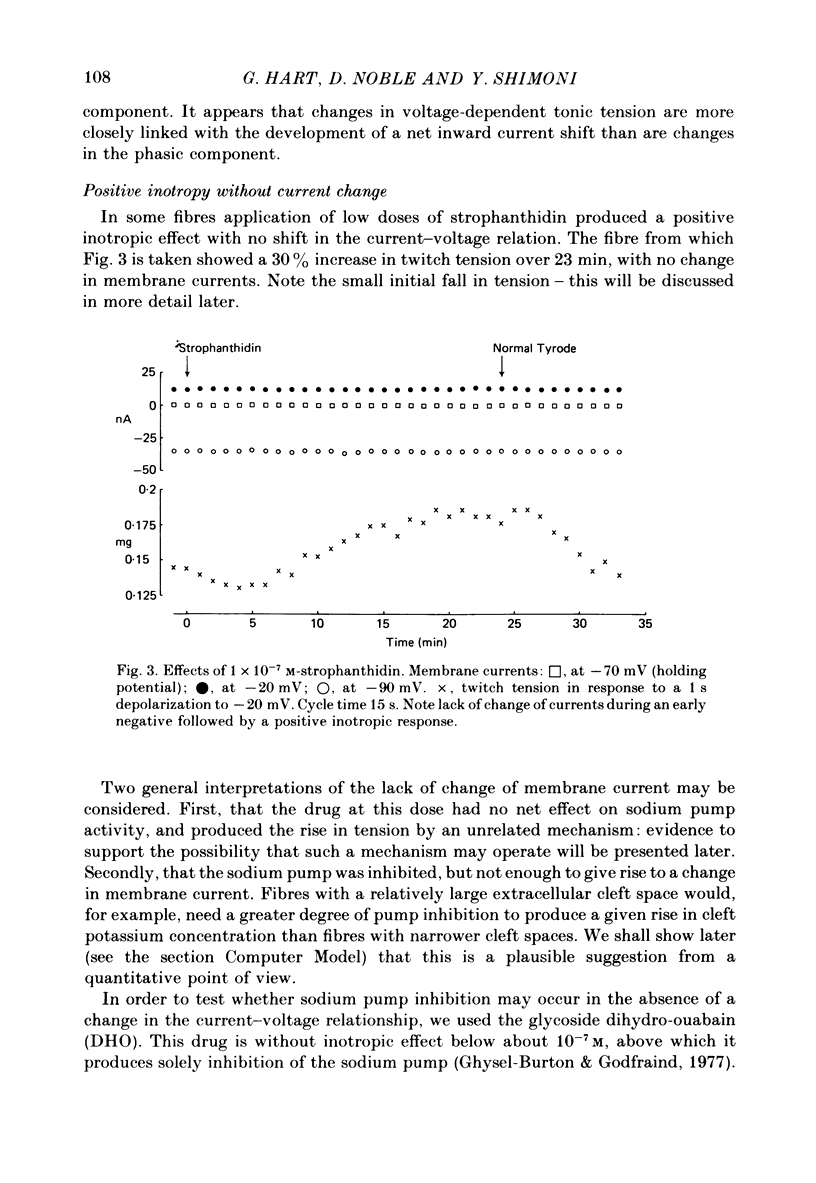
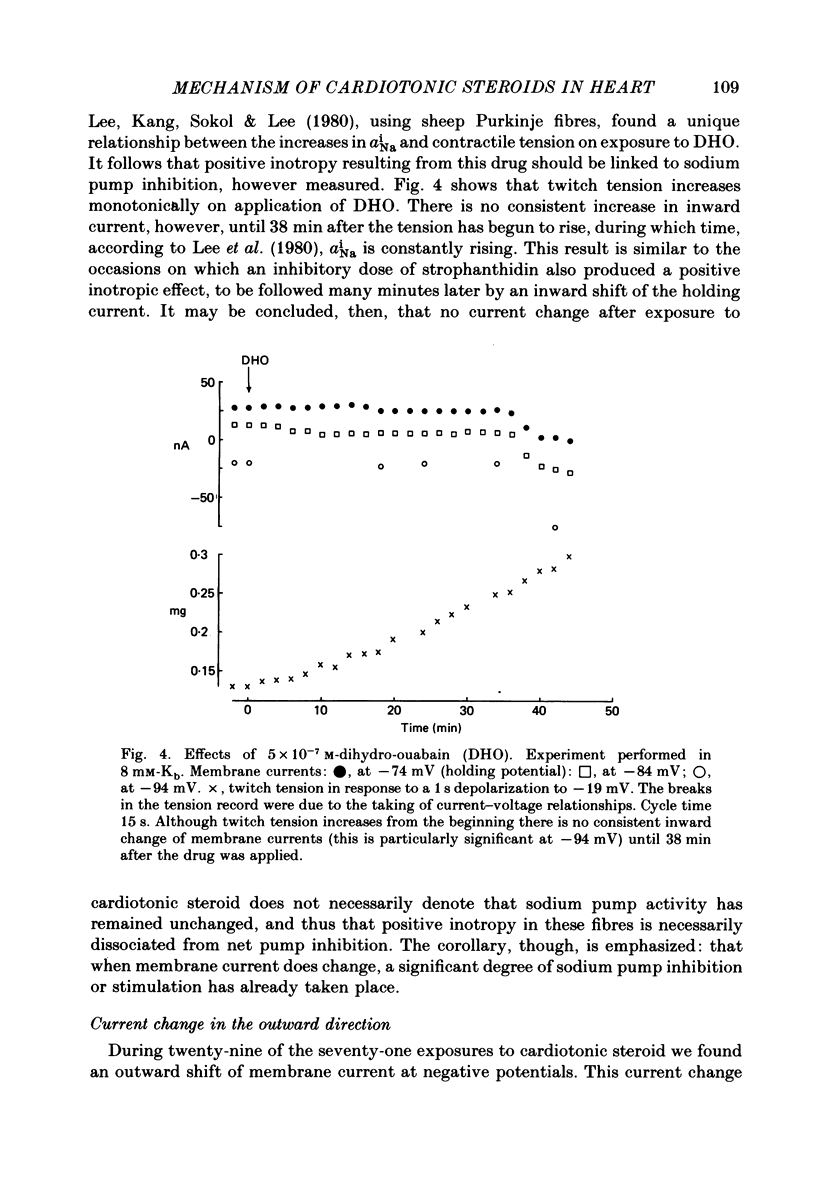
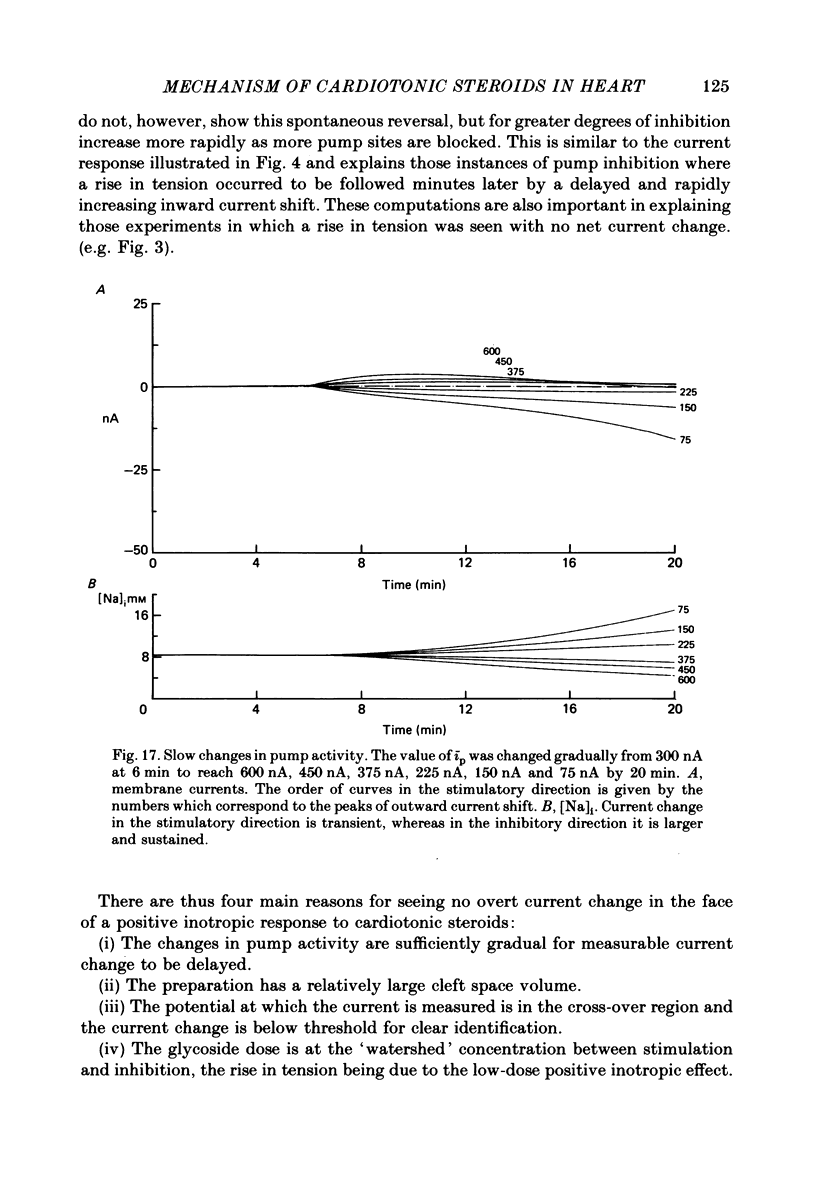
3. Exposure to dihydro-ouabain gave a monotonic increase in tension but a delayed increase in inward current. This suggests (cf. Lee, Kang, Sokol & Lee, 1980) that minor changes in pump activity may not always change the current—voltage relationship.
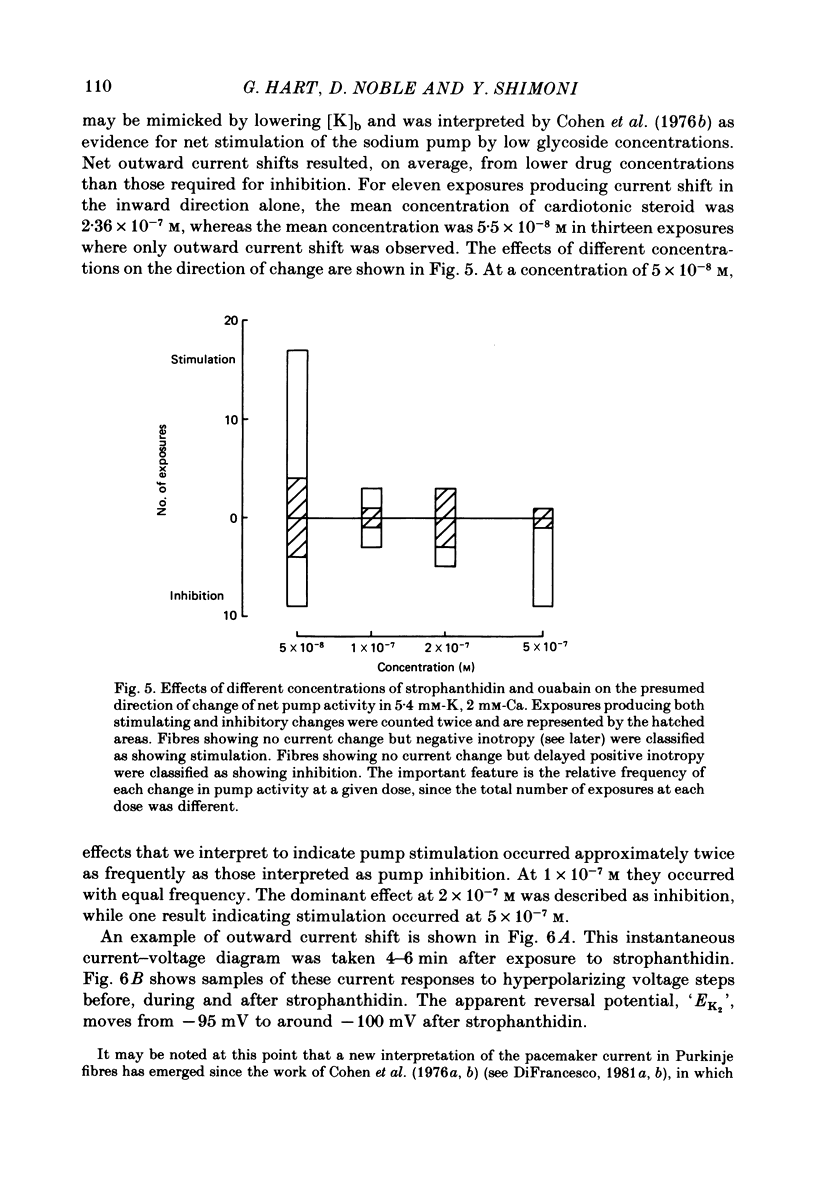
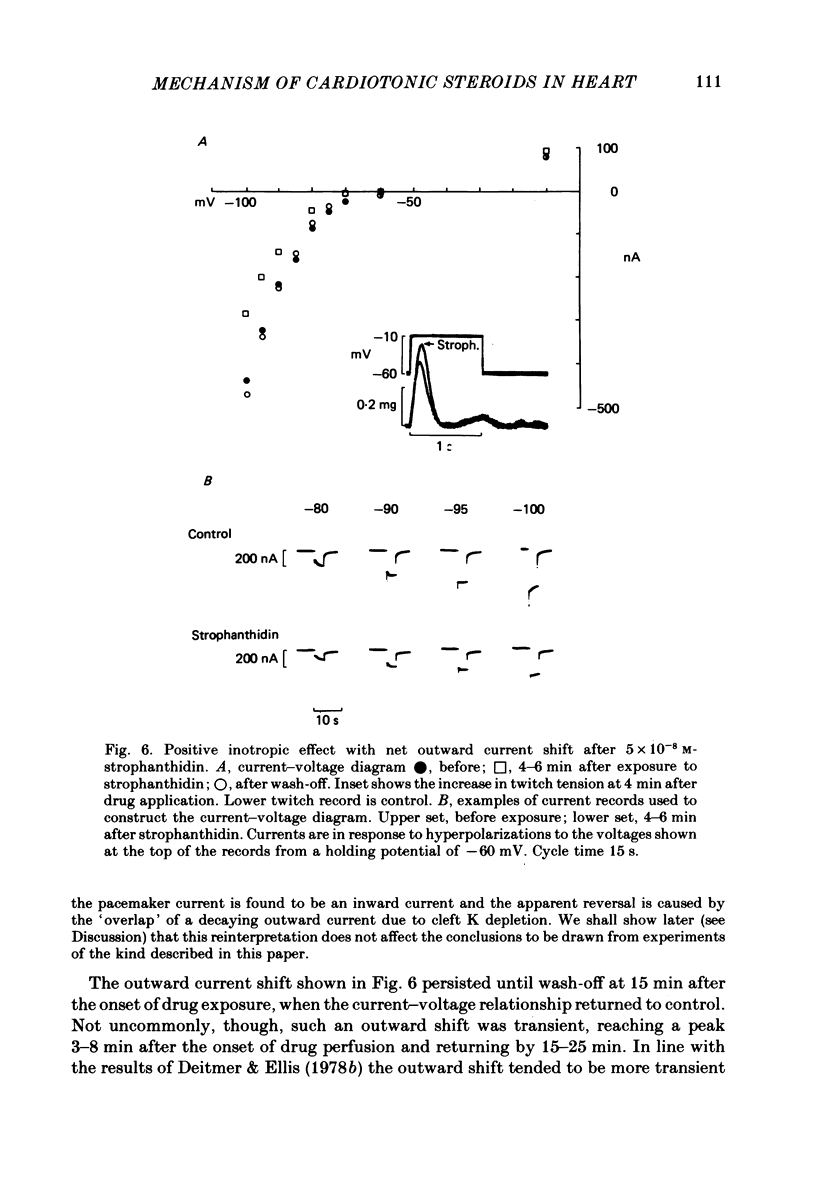
4. Low concentrations of strophanthidin (5 × 10-9 to 5 × 10-7 M) produced an outward current shift at very negative potentials, this change becoming smaller with a more rapid onset and reversing on increasing the dose. This change is attributed to pump stimulation.
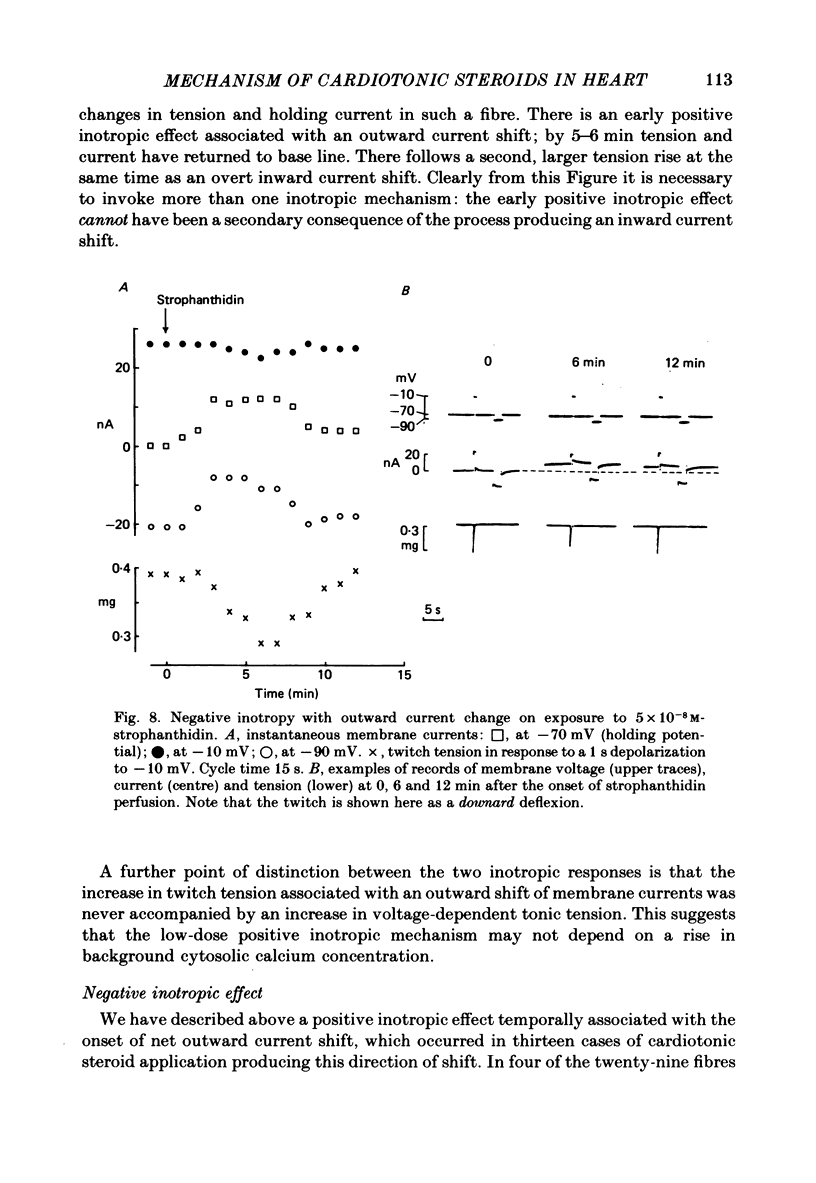
5. The outward current shift was often associated with a negative inotropic effect, which always reversed either spontaneously or on removal of the drug.
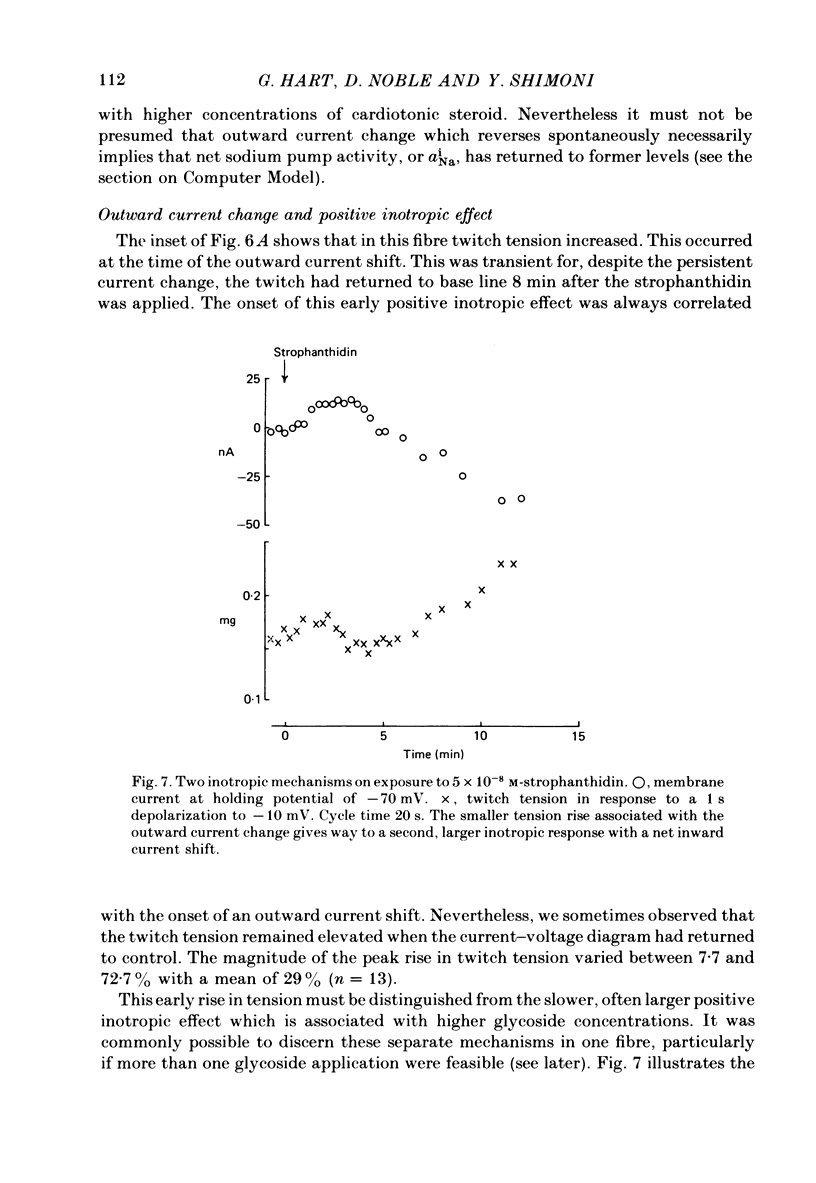
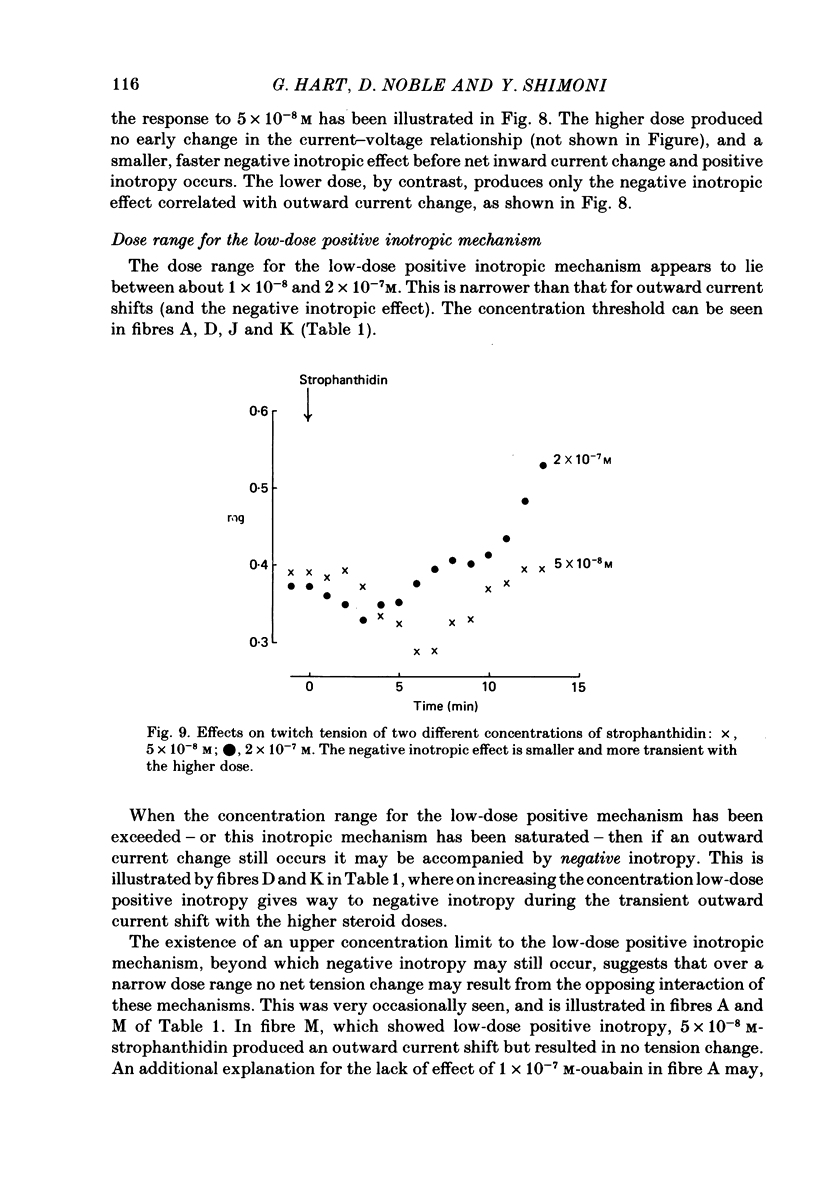
6. The alternative response at a narrower dose range (1 × 10-8 to 2 × 10-7 M) was an increase in twitch (not tonic) tension, termed the low-dose positive inotropic effect.
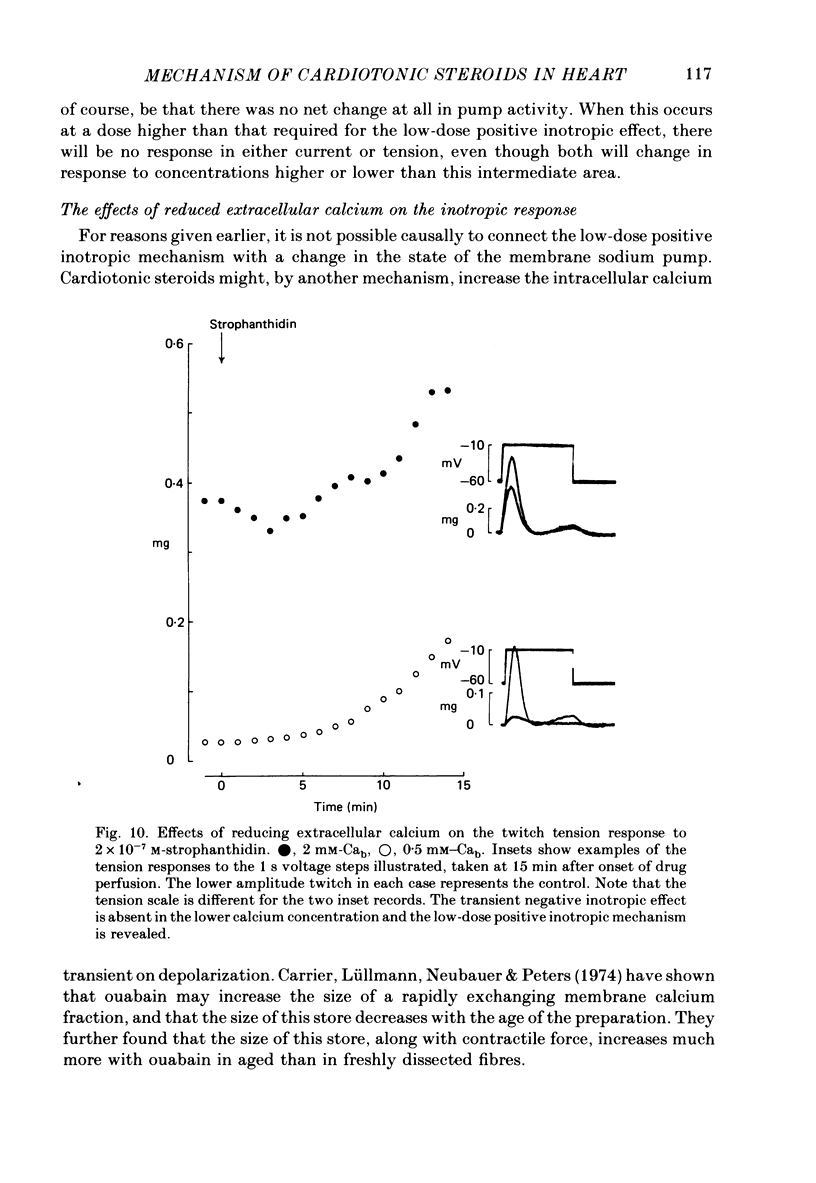
7. After a low concentration of cardiotonic steroid had given an early negative inotropic effect the bulk Ca concentration was reduced and the drug re-applied. The low-dose positive inotropic mechanism was then observed.
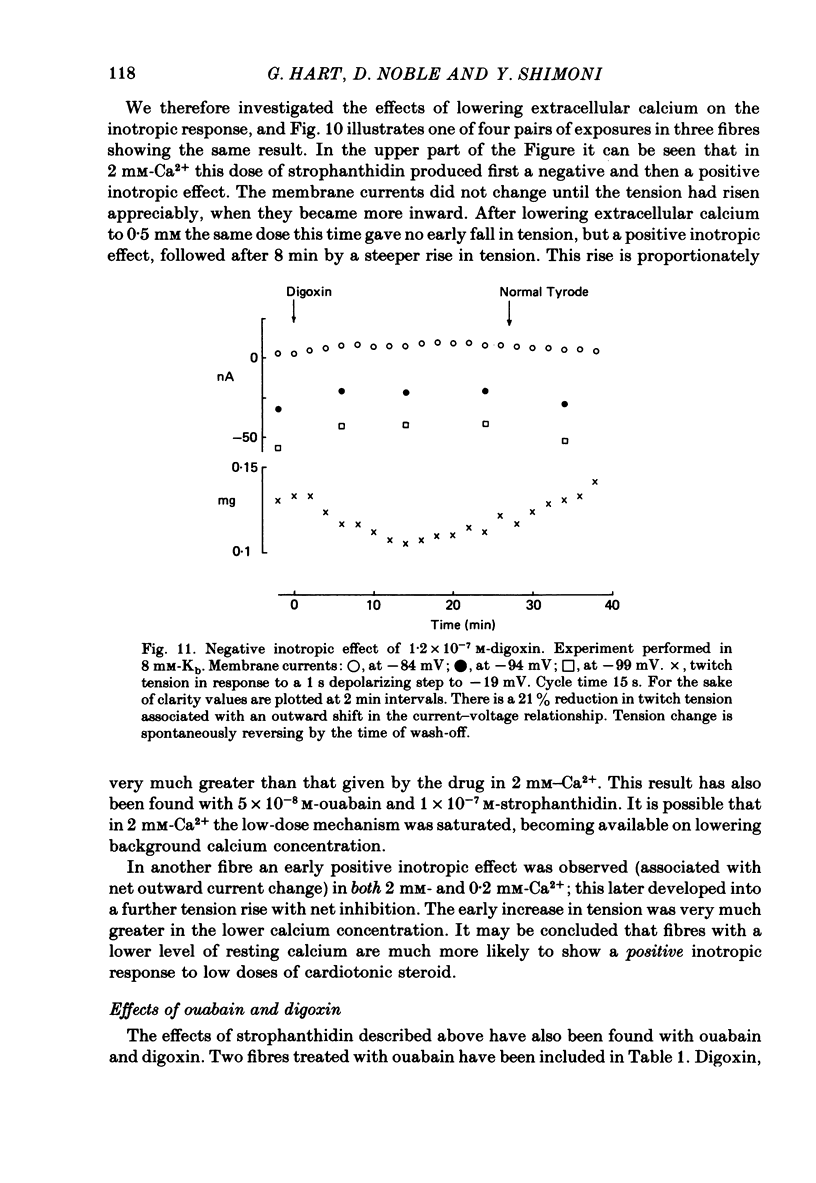
8. Outward current shifts and negative inotropy were also obtained with low concentrations of the clinically used glycosides digoxin and ouabain.
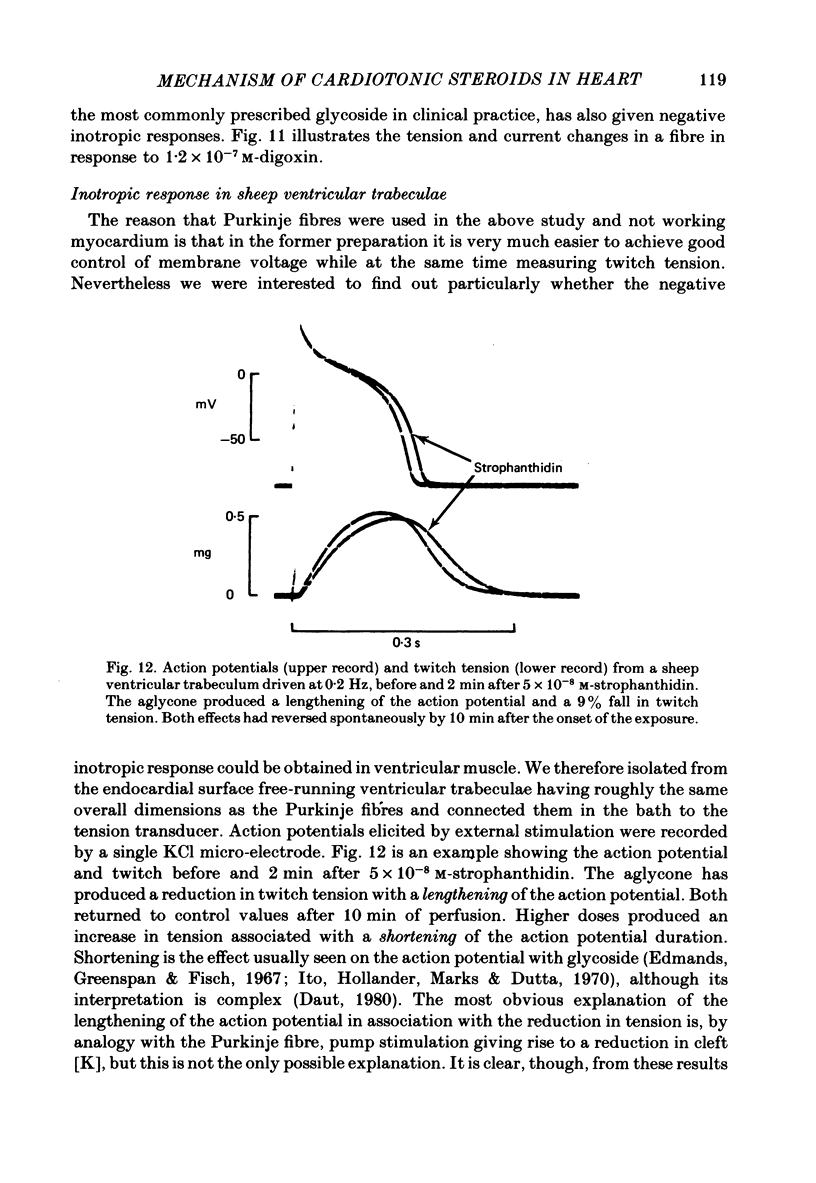
9. Low concentrations of strophanthidin applied to externally stimulated sheep ventricular trabeculae produced negative inotropy with lengthening of the action potential duration. Positive inotropy and action potential shortening occurred with higher doses.
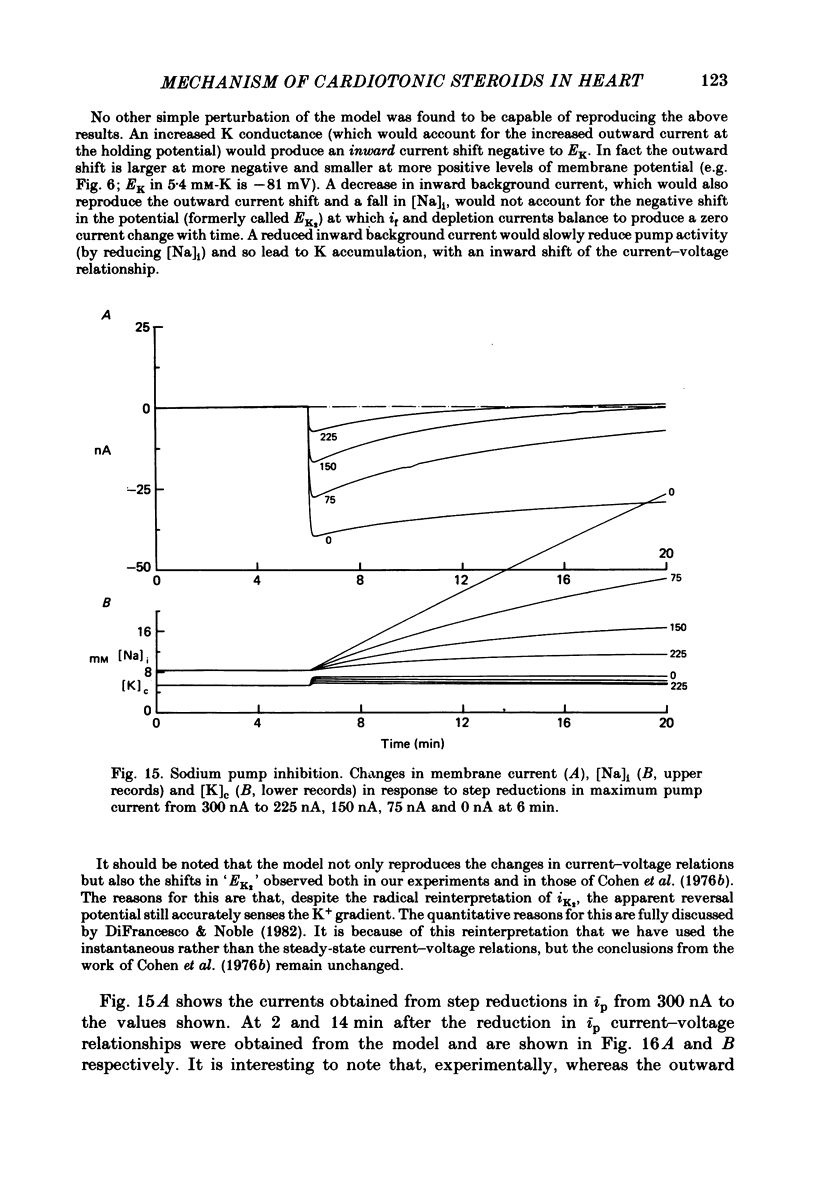
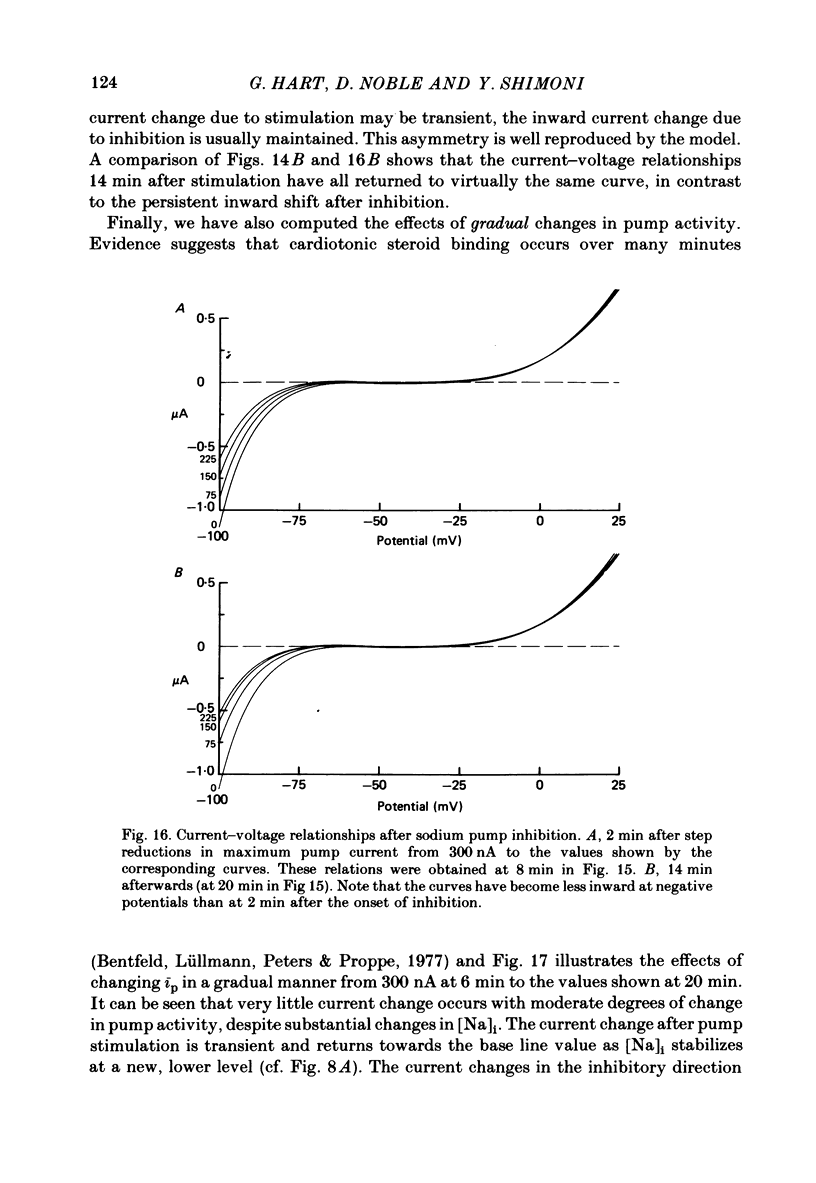
10. A computer model of ionic currents and distributions in Purkinje fibres satisfactorily reproduced the changes in membrane currents and ionic gradients observed with cardiotonic steroids. The only perturbations capable of explaining our results were Na pump stimulation and inhibition.
11. It is concluded that cardiotonic steroids possess two inotropic mechanisms. The first is a low-dose positive inotropic mechanism causally unrelated to changes in sodium pump activity and possibly a direct release of a membrane-associated calcium fraction. Should this mechanism be unavailable then net pump stimulation at low doses will produce negative inotropy. The second mechanism is the well known Na-lag process.
Full text
PDF











Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baker P. F., Blaustein M. P., Hodgkin A. L., Steinhardt R. A. The influence of calcium on sodium efflux in squid axons. J Physiol. 1969 Feb;200(2):431–458. doi: 10.1113/jphysiol.1969.sp008702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentfeld M., Lüllmann H., Peters T., Proppe D. Interdependence of ion transport and the action of quabain in heart muscle. Br J Pharmacol. 1977 Sep;61(1):19–27. doi: 10.1111/j.1476-5381.1977.tb09735.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrier G. O., Lüllmann H., Neubauer L., Peters T. The significance of a fast exchanging superficial calcium fraction for the regulation of contractile force in heart muscle. J Mol Cell Cardiol. 1974 Aug;6(4):333–347. doi: 10.1016/0022-2828(74)90075-3. [DOI] [PubMed] [Google Scholar]
- Cohen I., Daut J., Noble D. An analysis of the actions of low concentrations of ouabain on membrane currents in Purkinje fibres. J Physiol. 1976 Aug;260(1):75–103. doi: 10.1113/jphysiol.1976.sp011505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen I., Daut J., Noble D. The effects of potassium and temperature on the pace-maker current, iK2, in Purkinje fibres. J Physiol. 1976 Aug;260(1):55–74. doi: 10.1113/jphysiol.1976.sp011504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deitmer J. W., Ellis D. Changes in the intracellular sodium activity of sheep heart Purkinje fibres produced by calcium and other divalent cations. J Physiol. 1978 Apr;277:437–453. doi: 10.1113/jphysiol.1978.sp012283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deitmer J. W., Ellis D. The intracellular sodium activity of cardiac Purkinje fibres during inhibition and re-activation of the Na-K pump. J Physiol. 1978 Nov;284:241–259. doi: 10.1113/jphysiol.1978.sp012539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dempsey P. J., McCallum Z. T., Kent K. M., Cooper T. Dissociation of cardiac inotropic and transmembrane action potential effects of ouabain. J Pharmacol Exp Ther. 1971 Apr;177(1):78–84. [PubMed] [Google Scholar]
- DiFrancesco D. A new interpretation of the pace-maker current in calf Purkinje fibres. J Physiol. 1981 May;314:359–376. doi: 10.1113/jphysiol.1981.sp013713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiFrancesco D. A study of the ionic nature of the pace-maker current in calf Purkinje fibres. J Physiol. 1981 May;314:377–393. doi: 10.1113/jphysiol.1981.sp013714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edmonds R. E., Greenspan K., Fisch C. An electrophysiologic correlate of ouabain inotrophy in canine cardiac muscle. Circ Res. 1967 Oct;21(4):515–524. doi: 10.1161/01.res.21.4.515. [DOI] [PubMed] [Google Scholar]
- Eisner D. A., Lederer W. J. Inotropic and arrhythmogenic effects of potassium-depleted solutions on mammalian cardiac muscle. J Physiol. 1979 Sep;294:255–277. doi: 10.1113/jphysiol.1979.sp012929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisner D. A., Lederer W. J., Vaughan-Jones R. D. The dependence of sodium pumping and tension on intracellular sodium activity in voltage-clamped sheep Purkinje fibres. J Physiol. 1981 Aug;317:163–187. doi: 10.1113/jphysiol.1981.sp013819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis D. The effects of external cations and ouabain on the intracellular sodium activity of sheep heart Purkinje fibres. J Physiol. 1977 Dec;273(1):211–240. doi: 10.1113/jphysiol.1977.sp012090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erdmann E., Philipp G., Scholz H. Cardiac glycoside receptor, (Na+ + K+)-ATPase activity and force of contraction in rat heart. Biochem Pharmacol. 1980 Dec;29(24):3219–3229. doi: 10.1016/0006-2952(80)90295-6. [DOI] [PubMed] [Google Scholar]
- Gadsby D. C. Activation of electrogenic Na+/K+ exchange by extracellular K+ in canine cardiac Purkinje fibers. Proc Natl Acad Sci U S A. 1980 Jul;77(7):4035–4039. doi: 10.1073/pnas.77.7.4035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garnier D., Nargeot J., Ojeda C., Rougier O. The action of acetylcholine on background conductance in frog atrial trabeculae. J Physiol. 1978 Jan;274:381–396. doi: 10.1113/jphysiol.1978.sp012154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gervais A., Lane L. K., Anner B. M., Lindenmayer G. E., Schwartz A. A possible molecular mechanism of the action of digitalis: ouabain action on calcium binding to sites associated with a purified sodium-potassium-activated adenosine triphosphatase from kidney. Circ Res. 1977 Jan;40(1):8–14. doi: 10.1161/01.res.40.1.8. [DOI] [PubMed] [Google Scholar]
- Ghysel-Burton J., Godfraind T. Importance of the lactone ring for the action of therapeutic doses of ouabain in guinea-pig atria [proceedings]. J Physiol. 1977 Mar;266(1):75P–76P. [PubMed] [Google Scholar]
- Ghysel-Burton J., Godfraind T. Stimulation and inhibition of the sodium pump by cardioactive steroids in relation to their binding sites and their inotropic effect on guinea-pig isolated atria. Br J Pharmacol. 1979 Jun;66(2):175–184. doi: 10.1111/j.1476-5381.1979.tb13662.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giles W., Noble S. J. Changes in membrane currents in bullfrog atrium produced by acetylcholine. J Physiol. 1976 Sep;261(1):103–123. doi: 10.1113/jphysiol.1976.sp011550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUTTER O. F. Mode of action of autonomic transmitters on the heart. Br Med Bull. 1957 Sep;13(3):176–180. doi: 10.1093/oxfordjournals.bmb.a069609. [DOI] [PubMed] [Google Scholar]
- Hauswirth O., Noble D., Tsien R. W. Adrenaline: mechanism of action on the pacemaker potential in cardiac Purkinje fibers. Science. 1968 Nov 22;162(3856):916–917. doi: 10.1126/science.162.3856.916. [DOI] [PubMed] [Google Scholar]
- Hino N., Ochi R. Effect of acetylcholine on membrane currents in guinea-pig papillary muscle. J Physiol. 1980 Oct;307:183–197. doi: 10.1113/jphysiol.1980.sp013430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito M., Hollander P. B., Marks B. H., Dutta S. The effects of six cardiac glycosides on the transmembrane potential and contractile characteristics of the right ventricle of guinea pigs. J Pharmacol Exp Ther. 1970 Mar;172(1):188–195. [PubMed] [Google Scholar]
- Kass R. S., Lederer W. J., Tsien R. W., Weingart R. Role of calcium ions in transient inward currents and aftercontractions induced by strophanthidin in cardiac Purkinje fibres. J Physiol. 1978 Aug;281:187–208. doi: 10.1113/jphysiol.1978.sp012416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kass R. S., Tsien R. W., Weingart R. Ionic basis of transient inward current induced by strophanthidin in cardiac Purkinje fibres. J Physiol. 1978 Aug;281:209–226. doi: 10.1113/jphysiol.1978.sp012417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langer G. A., Serena S. D. Effects of strophanthidin upon contraction and ionic exchange in rabbit ventricular myocardium: relation to control of active state. J Mol Cell Cardiol. 1970 Mar;1(1):65–90. doi: 10.1016/0022-2828(70)90029-5. [DOI] [PubMed] [Google Scholar]
- Lee C. O., Kang D. H., Sokol J. H., Lee K. S. Relation between intracellular Na ion activity and tension of sheep cardiac Purkinje fibers exposed to dihydro-ouabain. Biophys J. 1980 Feb;29(2):315–330. doi: 10.1016/S0006-3495(80)85135-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nayler W. G. An effect of ouabain on the superficially-located stores of calcium in cardiac muscle cells. J Mol Cell Cardiol. 1973 Feb;5(1):101–110. doi: 10.1016/0022-2828(73)90039-4. [DOI] [PubMed] [Google Scholar]
- Noble D. Mechanism of action of therapeutic levels of cardiac glycosides. Cardiovasc Res. 1980 Sep;14(9):495–514. doi: 10.1093/cvr/14.9.495. [DOI] [PubMed] [Google Scholar]
- Noma A., Trautwein W. Relaxation of the ACh-induced potassium current in the rabbit sinoatrial node cell. Pflugers Arch. 1978 Nov 30;377(3):193–200. doi: 10.1007/BF00584272. [DOI] [PubMed] [Google Scholar]
- Ojeda C., Rougier O., Tourneur Y. Effects of Cs on acetylcholine induced current. Is ik1 increased by acetylcholine in frog atrium? Pflugers Arch. 1981 Jul;391(1):57–59. doi: 10.1007/BF00580695. [DOI] [PubMed] [Google Scholar]
- Peters T., Raben R. H., Wassermann O. Evidence for a dissociation between positive inotropic effect and inhibition of the Na+-K+-ATPase by ouabain, cassaine and their alkylating derivatives. Eur J Pharmacol. 1974 May;26(2):166–174. doi: 10.1016/0014-2999(74)90223-4. [DOI] [PubMed] [Google Scholar]
- Poole-Wilson P. A., Galindez E., Fry C. H. Effect of ouabain in therapeutic concentrations on K+ exchange and contraction of human and rabbit myocardium. Clin Sci (Lond) 1979 Nov;57(5):415–420. doi: 10.1042/cs0570415. [DOI] [PubMed] [Google Scholar]
- Reuter H., Scholz H. A study of the ion selectivity and the kinetic properties of the calcium dependent slow inward current in mammalian cardiac muscle. J Physiol. 1977 Jan;264(1):17–47. doi: 10.1113/jphysiol.1977.sp011656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuter H., Seitz N. The dependence of calcium efflux from cardiac muscle on temperature and external ion composition. J Physiol. 1968 Mar;195(2):451–470. doi: 10.1113/jphysiol.1968.sp008467. [DOI] [PMC free article] [PubMed] [Google Scholar]


