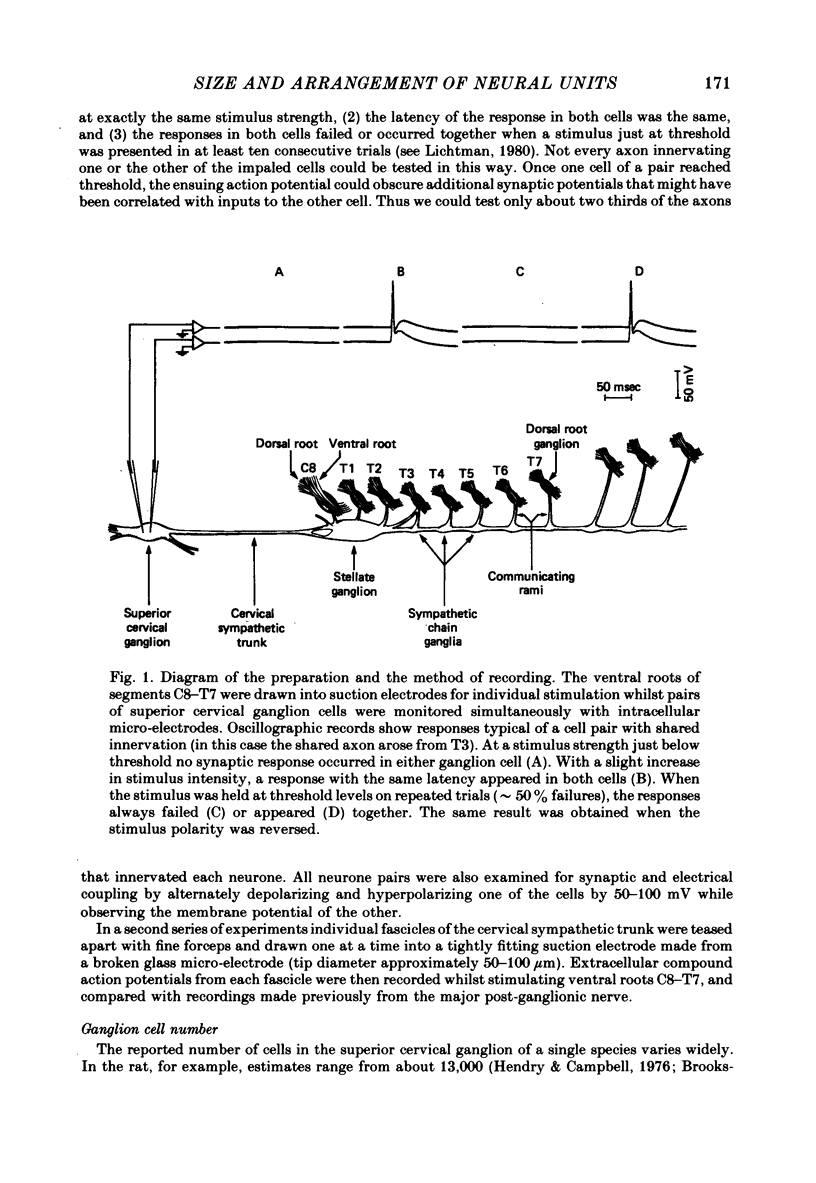
Abstract
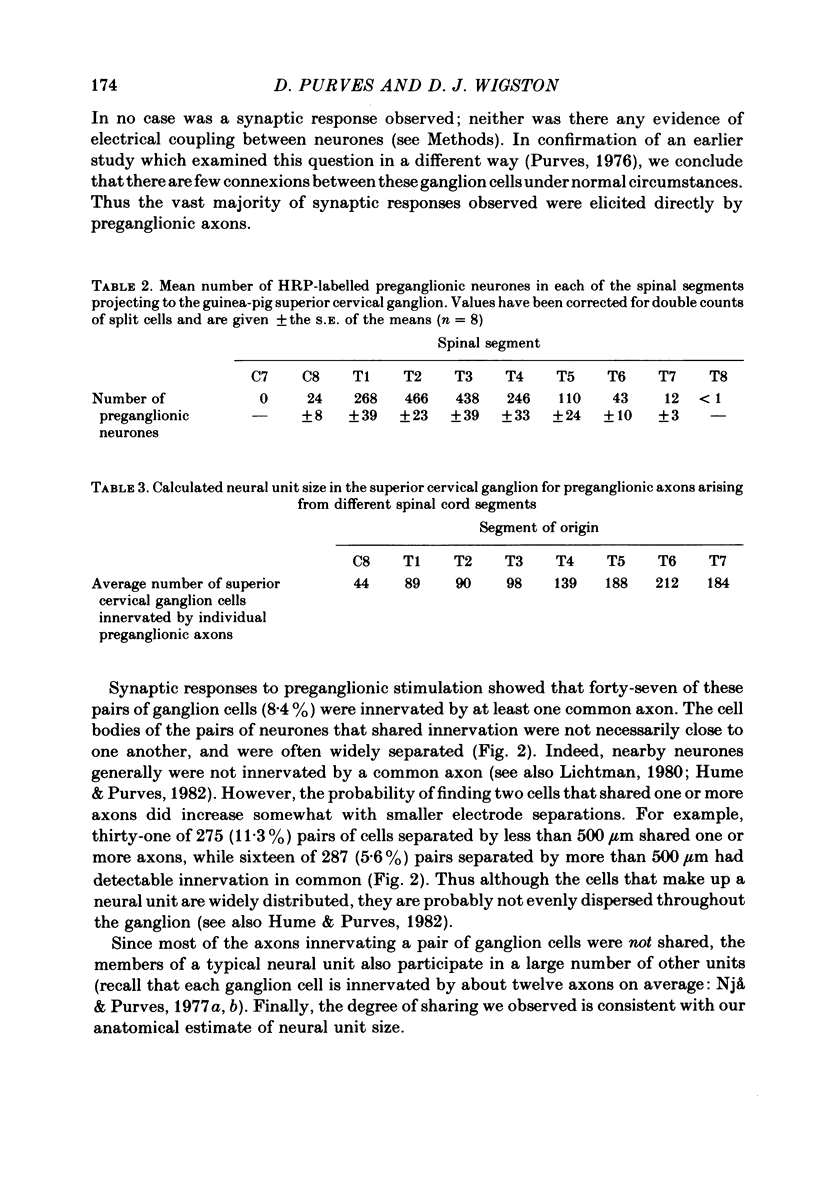
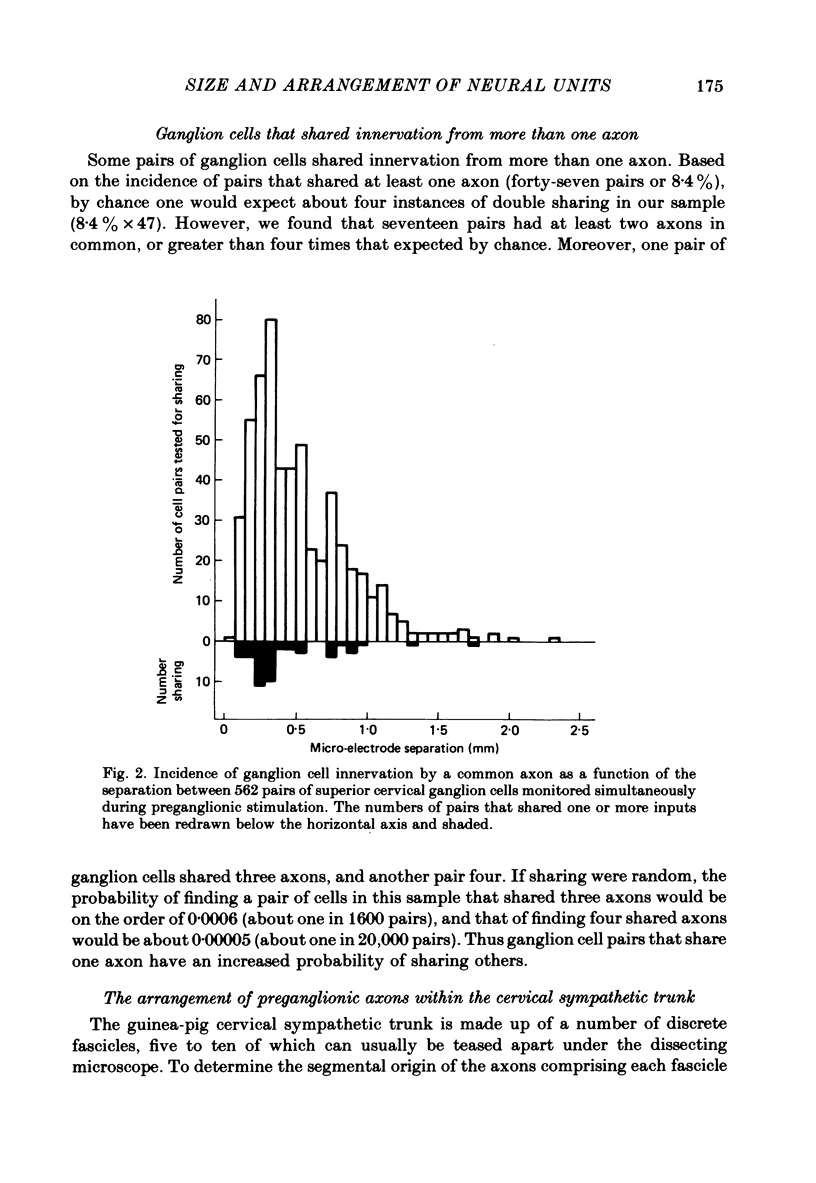
The size and arrangement of the set of neurones innervated by individual preganglionic axons (the neural unit) has been investigated in the superior cervical ganglion of the guinea-pig. 1. Based on the ratio of preganglionic neurones to ganglion cells, and the average number of axons contacting each ganglion cell, we estimated that individual preganglionic axons innervate on the order of 50-200 superior cervical ganglion cells. 2. Of 562 pairs of ganglion cells examined with intracellular recording, forty-seven (8.4%) were innervated by one or more common axons. 3. Pairs of ganglion cells innervated by the same axon were not necessarily near each other. Although nearby cells were more likely to share innervation than neurones far apart, cells sharing innervation were often found several hundred micrometers apart, and were occasionally separated by the largest dimension of the ganglion (about 1-2 mm). 4. The incidence of cell pairs that shared innervation from more than one axon was greater than expected from the frequency of pairs sharing at least one axon. 5. Extracellular recordings from small fascicles of the cervical sympathetic trunk showed that preganglionic axons from different segmental levels intermingle extensively en route to the superior cervical ganglion. 6. Taken together, these findings support the view that sets of ganglion cells are innervated in common not because of any special topographic relationship within the ganglion, but because they share one or more properties that make them especially attractive to particular preganglionic axons.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bowers C. W., Zigmond R. E. Sympathetic neurons in lower cervical ganglia send axons through the superior cervical ganglion. Neuroscience. 1981;6(9):1783–1791. doi: 10.1016/0306-4522(81)90213-x. [DOI] [PubMed] [Google Scholar]
- Brooks-Fournier R., Coggeshall R. E. The ratio of preganglionic axons to postganglionic cells in the sympathetic nervous system of the rat. J Comp Neurol. 1981 Apr 1;197(2):207–216. doi: 10.1002/cne.901970204. [DOI] [PubMed] [Google Scholar]
- Burke R. E., Levine D. N., Salcman M., Tsairis P. Motor units in cat soleus muscle: physiological, histochemical and morphological characteristics. J Physiol. 1974 May;238(3):503–514. doi: 10.1113/jphysiol.1974.sp010540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke R. E., Tsairis P. Anatomy and innervation ratios in motor units of cat gastrocnemius. J Physiol. 1973 Nov;234(3):749–765. doi: 10.1113/jphysiol.1973.sp010370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalsgaard C. J., Elfvin L. G. Spinal origin of preganglionic fibers projecting onto the superior cervical ganglion and inferior mesenteric ganglion of the guinea pig, as demonstrated by the horseradish peroxidase technique. Brain Res. 1979 Aug 17;172(1):139–143. doi: 10.1016/0006-8993(79)90901-6. [DOI] [PubMed] [Google Scholar]
- Hendry I. A., Campbell J. Morphometric analysis of rat superior cervical ganglion after axotomy and nerve growth factor treatment. J Neurocytol. 1976 Jun;5(3):351–360. doi: 10.1007/BF01175120. [DOI] [PubMed] [Google Scholar]
- Johnson E. M., Jr, Gorin P. D., Brandeis L. D., Pearson J. Dorsal root ganglion neurons are destroyed by exposure in utero to maternal antibody to nerve growth factor. Science. 1980 Nov 21;210(4472):916–918. doi: 10.1126/science.7192014. [DOI] [PubMed] [Google Scholar]
- Lichtman J. W. On the predominantly single innervation of submandibular ganglion cells in the rat. J Physiol. 1980 May;302:121–130. doi: 10.1113/jphysiol.1980.sp013233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtman J. W., Purves D., Yip J. W. Innervation of sympathetic neurones in the guinea-pig thoracic chain. J Physiol. 1980 Jan;298:285–299. doi: 10.1113/jphysiol.1980.sp013081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtman J. W., Purves D., Yip J. W. On the purpose of selective innervation of guinea-pig superior cervical ganglion cells. J Physiol. 1979 Jul;292:69–84. doi: 10.1113/jphysiol.1979.sp012839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maehlen J., Njå A. Selective synapse formation during sprouting after partial denervation of the guinea-pig superior cervical ganglion. J Physiol. 1981;319:555–567. doi: 10.1113/jphysiol.1981.sp013926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLachlan E. M. The formation of synapses in mammalian sympathetic ganglia reinnervated with preganglionic or somatic nerves. J Physiol. 1974 Feb;237(1):217–242. doi: 10.1113/jphysiol.1974.sp010479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nja A., Purves D. Re-innervation of guinea-pig superior cervical ganglion cells by preganglionic fibres arising from different levels of the spinal cord. J Physiol. 1977 Nov;272(3):633–651. doi: 10.1113/jphysiol.1977.sp012064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Njå A., Purves D. Specific innervation of guinea-pig superior cervical ganglion cells by preganglionic fibres arising from different levels of the spinal cord. J Physiol. 1977 Jan;264(2):565–583. doi: 10.1113/jphysiol.1977.sp011683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Njå A., Purves D. Specificity of initial synaptic contacts made on guinea-pig superior cervical ganglion cells during regeneration of the cervical sympathetic trunk. J Physiol. 1978 Aug;281:45–62. doi: 10.1113/jphysiol.1978.sp012408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perri V., Sacchi O., Casella C. Synaptically mediated potentials elicited by the stimulation of post-ganglionic trunks in the guinea-pig superior cervical ganglion. Pflugers Arch. 1970;314(1):55–67. doi: 10.1007/BF00587046. [DOI] [PubMed] [Google Scholar]
- Purves D. Competitive and non-competitive re-innervation of mammalian sympathetic neurones by native and foreign fibres. J Physiol. 1976 Oct;261(2):453–475. doi: 10.1113/jphysiol.1976.sp011568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purves D. Functional and structural changes in mammalian sympathetic neurones following interruption of their axons. J Physiol. 1975 Nov;252(2):429–463. doi: 10.1113/jphysiol.1975.sp011151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purves D., Lichtman J. W. Formation and maintenance of synaptic connections in autonomic ganglia. Physiol Rev. 1978 Oct;58(4):821–862. doi: 10.1152/physrev.1978.58.4.821. [DOI] [PubMed] [Google Scholar]
- Purves D., Thompson W. The effects of post-ganglionic axotomy on selective synaptic connexions in the superior cervical ganglion of the guinea-pig. J Physiol. 1979 Dec;297(0):95–110. doi: 10.1113/jphysiol.1979.sp013029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rando T. A., Bowers C. W., Zigmond R. E. Localization of neurons in the rat spinal cord which project to the superior cervical ganglion. J Comp Neurol. 1981 Feb 10;196(1):73–83. doi: 10.1002/cne.901960107. [DOI] [PubMed] [Google Scholar]
- Rubin E., Purves D. Segmental organization of sympathetic preganglionic neurons in the mammalian spinal cord. J Comp Neurol. 1980 Jul 1;192(1):163–174. doi: 10.1002/cne.901920111. [DOI] [PubMed] [Google Scholar]
- de Olmos J., Hardy H., Heimer L. The afferent connections of the main and the accessory olfactory bulb formations in the rat: an experimental HRP-study. J Comp Neurol. 1978 Sep 15;181(2):213–244. doi: 10.1002/cne.901810202. [DOI] [PubMed] [Google Scholar]


