Abstract
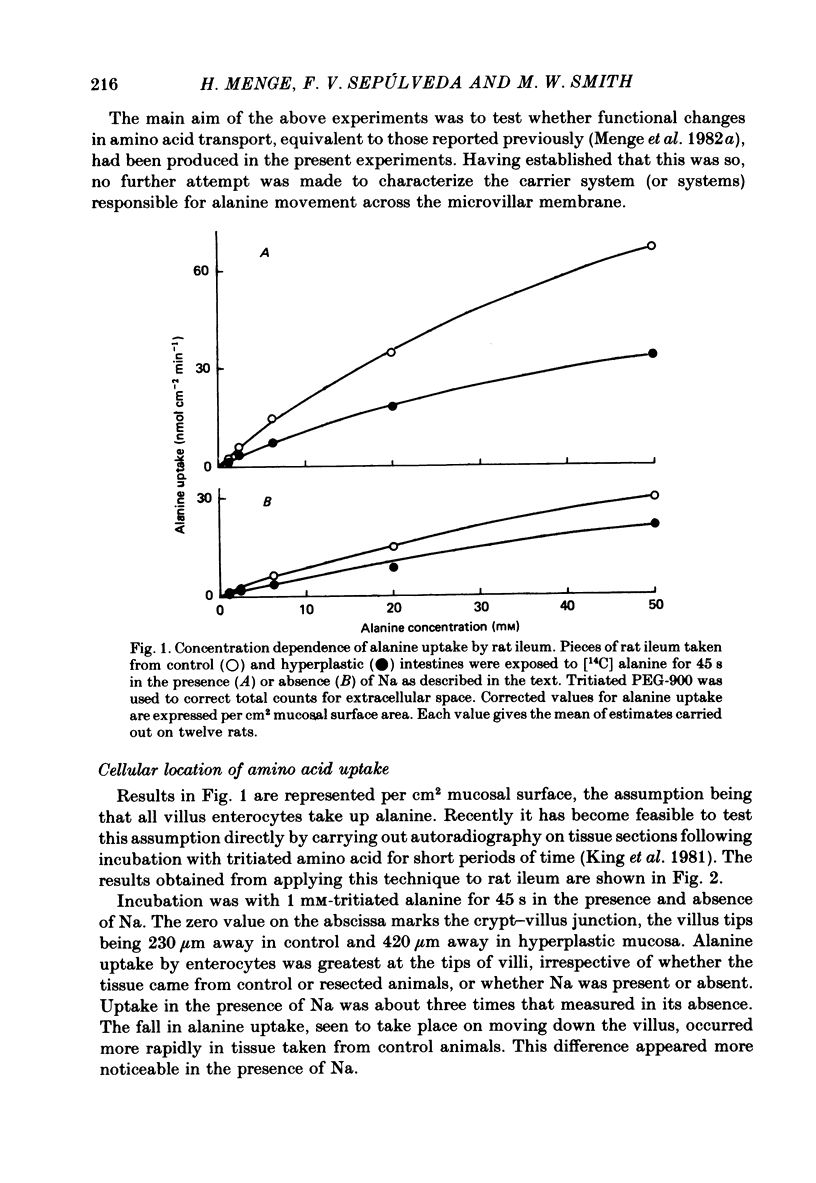
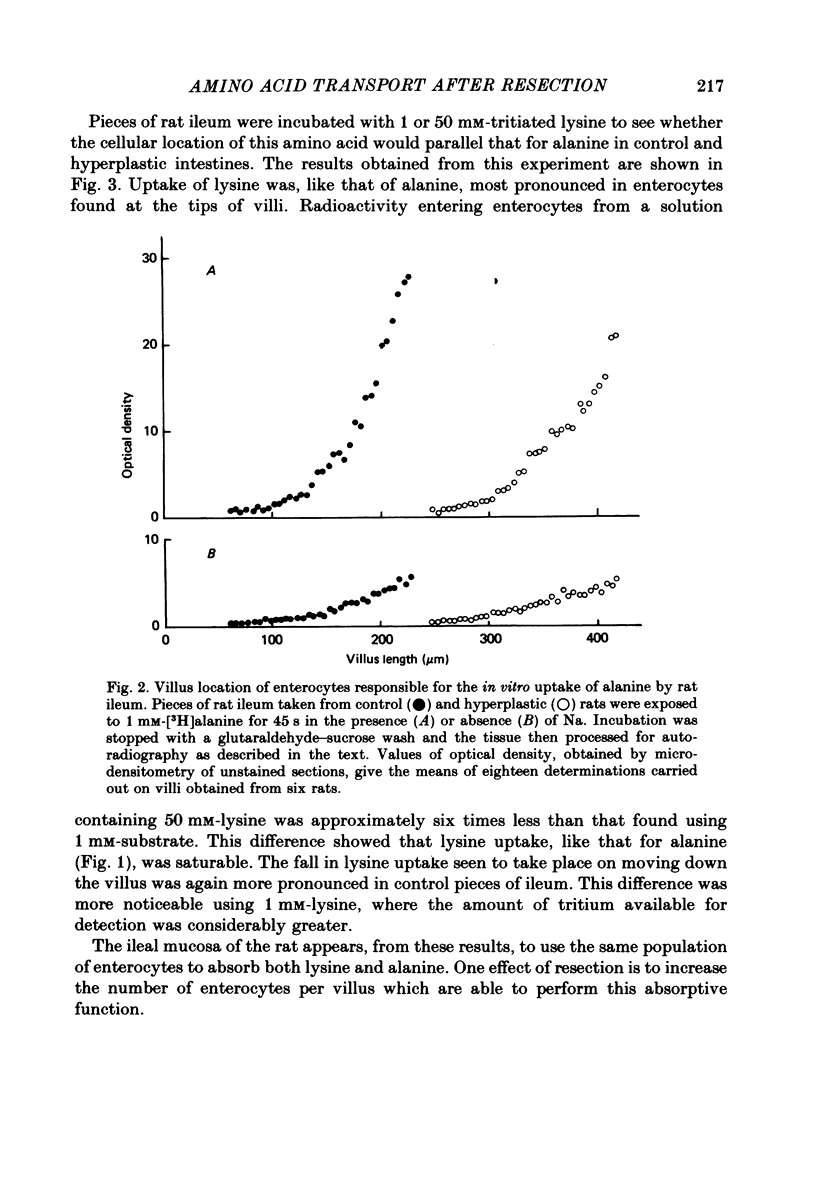
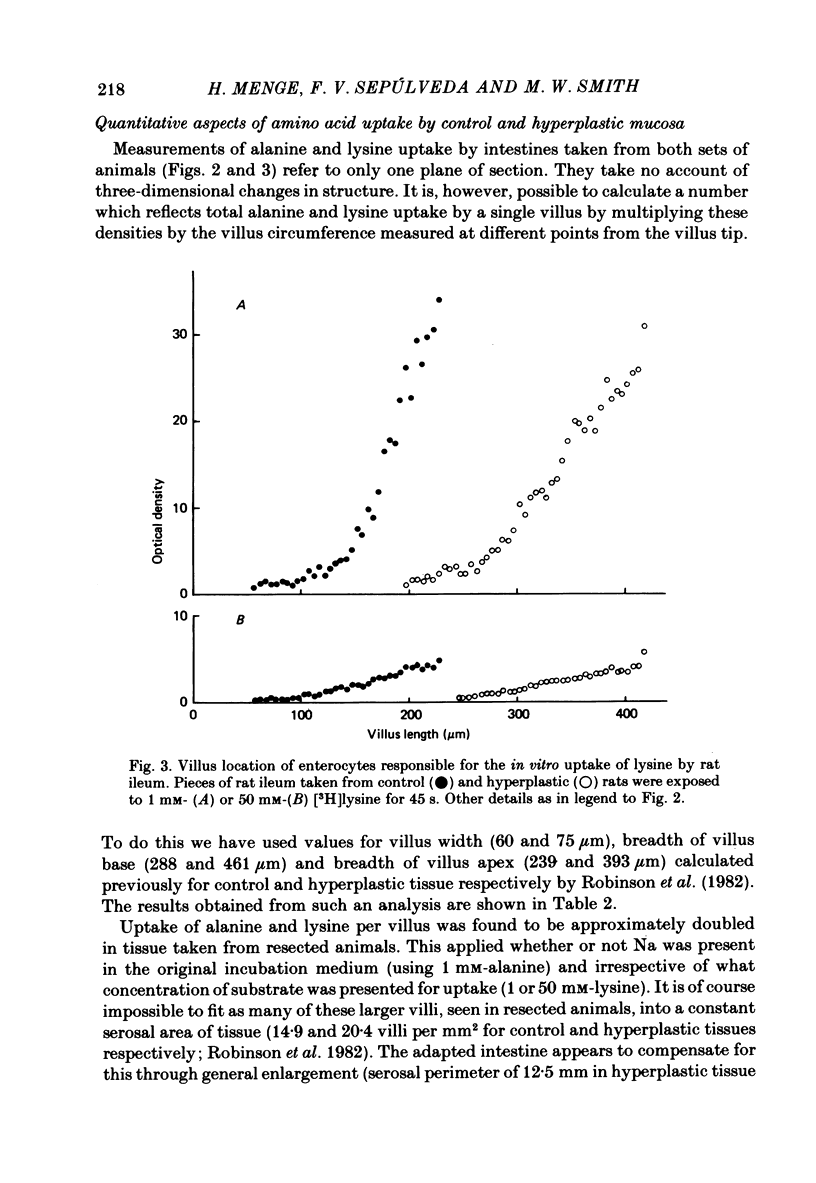
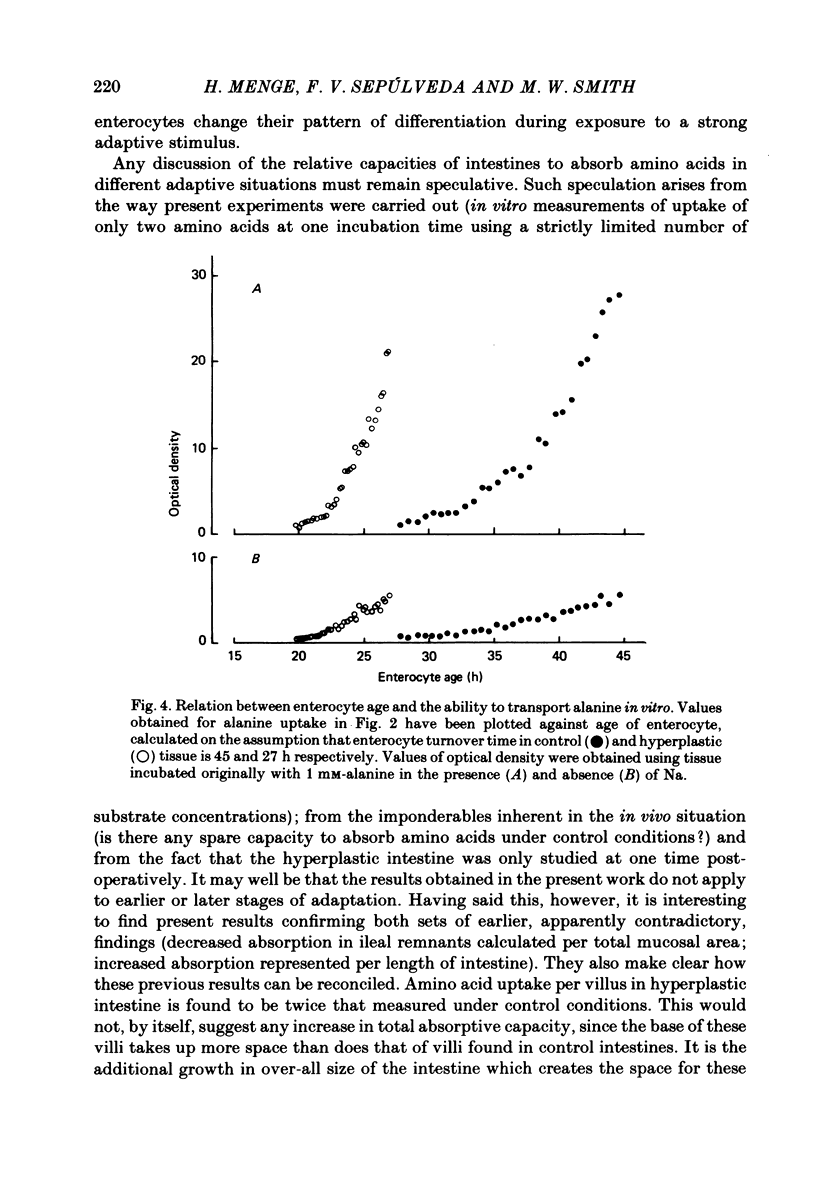
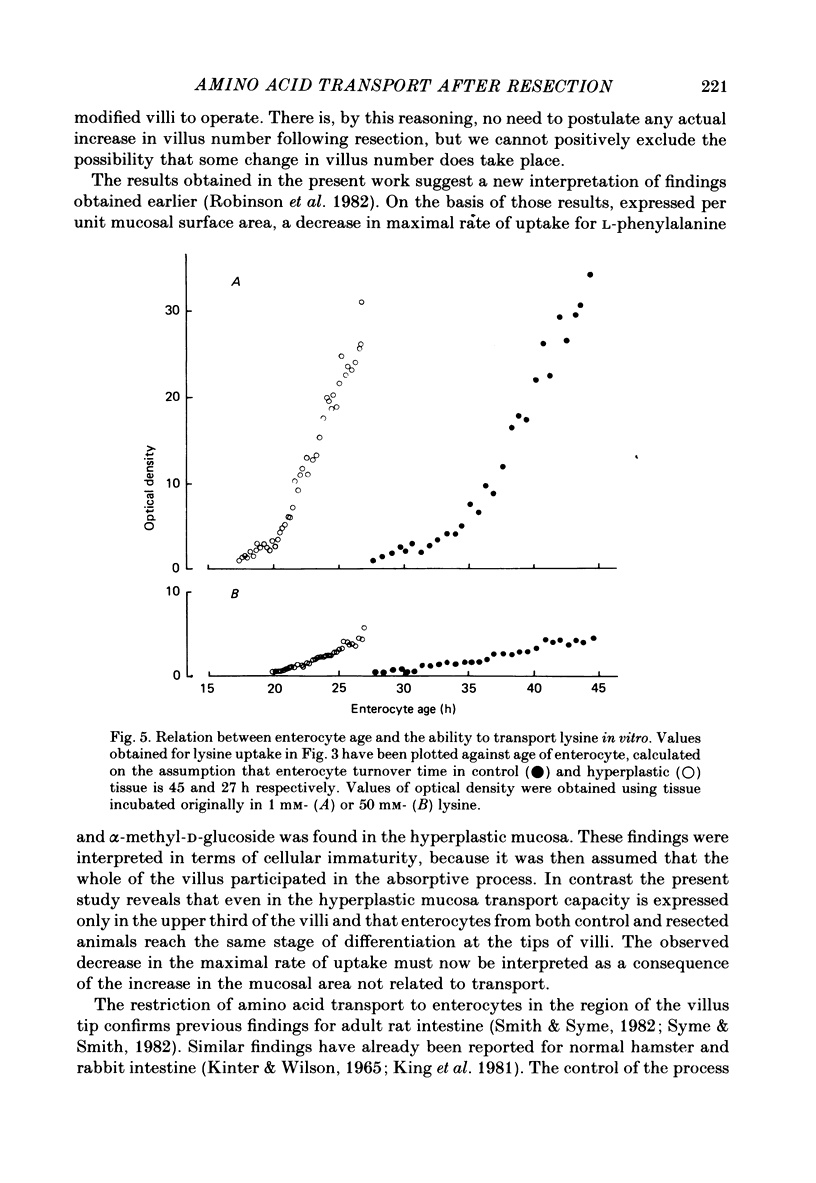
1. The ability of rat ileal enterocytes to take up alanine and lysine before and after proximal resection of the small intestine has been assessed using both autoradiographic and dual-isotope methods of analysis. 2. The length of individual villi was approximately doubled after resection. Alanine uptake measured in the presence or absence of Na, represented per cm2 mucosal surface area, decreased following intestinal resection. 3. Alanine and lysine uptake were confined to villus tip enterocytes in both control and ileal remnants. The net effect of intestinal resection was to increase amino acid uptake calculated per individual villus or per unit length of intestine. 4. Adaptational changes occurring as a result of resection included a shortening of the time needed for enterocytes to reach the stage where they first began to absorb amino acids and a doubling of the rate at which absorption increased during the later stages of enterocyte differentiation. 5. It is suggested that the physiological response to intestinal resection can best be appreciated by studying events taking place within individual enterocytes. The way in which adaptational changes become organized within the mucosa remains to be elucidated.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BOOTH C. C., EVANS K. T., MENZIES T., STREET D. F. Intestinal hypertrophy following partial resection of the small bowel in the rat. Br J Surg. 1959 Jan;46(198):403–410. doi: 10.1002/bjs.18004619821. [DOI] [PubMed] [Google Scholar]
- Dowling R. H., Booth C. C. Structural and functional changes following small intestinal resection in the rat. Clin Sci. 1967 Feb;32(1):139–149. [PubMed] [Google Scholar]
- Garrido A. B., Jr, Freeman H. J., Chung Y. C., Kim Y. S. Amino acid and peptide absorption after proximal small intestinal resection in the rat. Gut. 1979 Feb;20(2):114–120. doi: 10.1136/gut.20.2.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutschmidt S., Emde C. Early changes in brush border disaccharidase kinetics in rat jejunum following subcutaneous administration of tetraiodothyronine: a quantitative histochemical study on villi revealing normal morphology. Histochemistry. 1981;73(1):151–160. doi: 10.1007/BF00493141. [DOI] [PubMed] [Google Scholar]
- Hanson W. R., Osborne J. W. Epithelial cell kinetics in the small intestine of the rat 60 days after resection of 70 per cent of the ileum and jejunum. Gastroenterology. 1971 Jun;60(6):1087–1097. [PubMed] [Google Scholar]
- King I. S., Sepúlveda F. V., Smith M. W. Cellular distribution of neutral and basic amino acid transport systems in rabbit ileal mucosa. J Physiol. 1981;319:355–368. doi: 10.1113/jphysiol.1981.sp013913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LORAN M. R., ALTHAUSEN T. L. Hypertrophy and changes in cholinesterase activities of the intestine, erythrocytes and plasma after partial resection of the small intestine of the rat. Am J Physiol. 1958 Jun;193(3):516–520. doi: 10.1152/ajplegacy.1958.193.3.516. [DOI] [PubMed] [Google Scholar]
- LORAN M. R., CROCKER T. T. POPULATION DYNAMICS OF INTESTINAL EPITHELIA IN THE RAT TWO MONTHS AFTER PARTIAL RESECTION OF THE ILEUM. J Cell Biol. 1963 Nov;19:285–291. doi: 10.1083/jcb.19.2.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menge H., Murer H., Robinson J. W. Glucose transport by brush-border membrane vesicles after proximal resection or ileo-jejunal transposition in the rat. J Physiol. 1978 Jan;274:9–16. doi: 10.1113/jphysiol.1978.sp012130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menge H., Robinson J. W., Riecken E. O. Anpassungsmöglichkeiten der Dünndarmschleimhaut an verschiedene intraluminale Milieuveränderungen. Z Gastroenterol. 1976 May;14(3):420–433. [PubMed] [Google Scholar]
- Menge H., Robinson J. W. The relationship between the functional and structural alterations in the rat small intestine following proximal resection of varying extents. Res Exp Med (Berl) 1978 Jul 24;173(1):41–53. doi: 10.1007/BF01851373. [DOI] [PubMed] [Google Scholar]
- Sepúlveda F. V., Burton K. A., Clarkson G. M., Syme G. Cell differentiation and L-ornithine decarboxylase activity in the small intestine of rats fed low and high protein diets. Biochim Biophys Acta. 1982 Jun 16;716(3):439–442. doi: 10.1016/0304-4165(82)90038-1. [DOI] [PubMed] [Google Scholar]
- Smith M. W., Syme G. Functional differentiation of enterocytes in the follicle-associated epithelium of rat Peyer's patch. J Cell Sci. 1982 Jun;55:147–156. doi: 10.1242/jcs.55.1.147. [DOI] [PubMed] [Google Scholar]
- Syme G., Smith M. W. Intestinal adaptation of protein deficiency. Cell Biol Int Rep. 1982 Jun;6(6):573–578. doi: 10.1016/0309-1651(82)90181-3. [DOI] [PubMed] [Google Scholar]
- Syme G. The effect of protein-deficient isoenergetic diets on the growth of rat jejunal mucosa. Br J Nutr. 1982 Jul;48(1):25–36. doi: 10.1079/bjn19820084. [DOI] [PubMed] [Google Scholar]


