Abstract
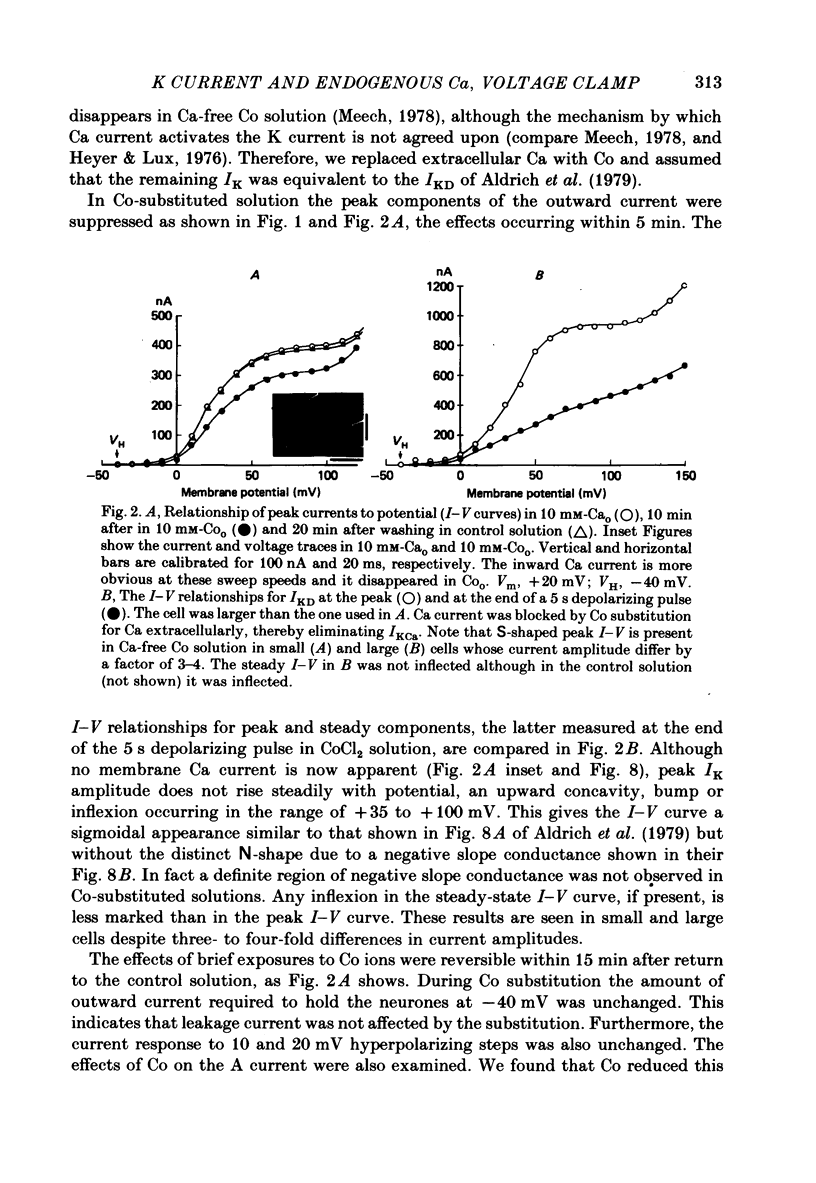
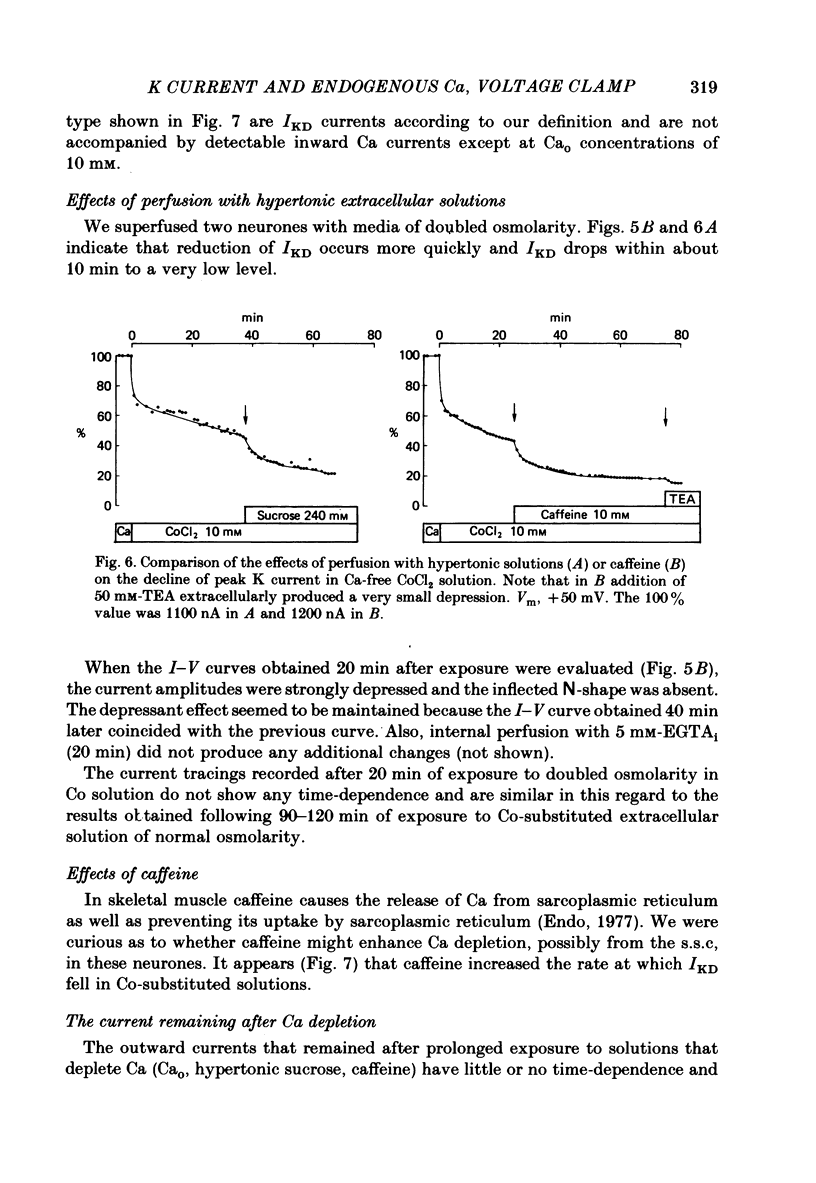
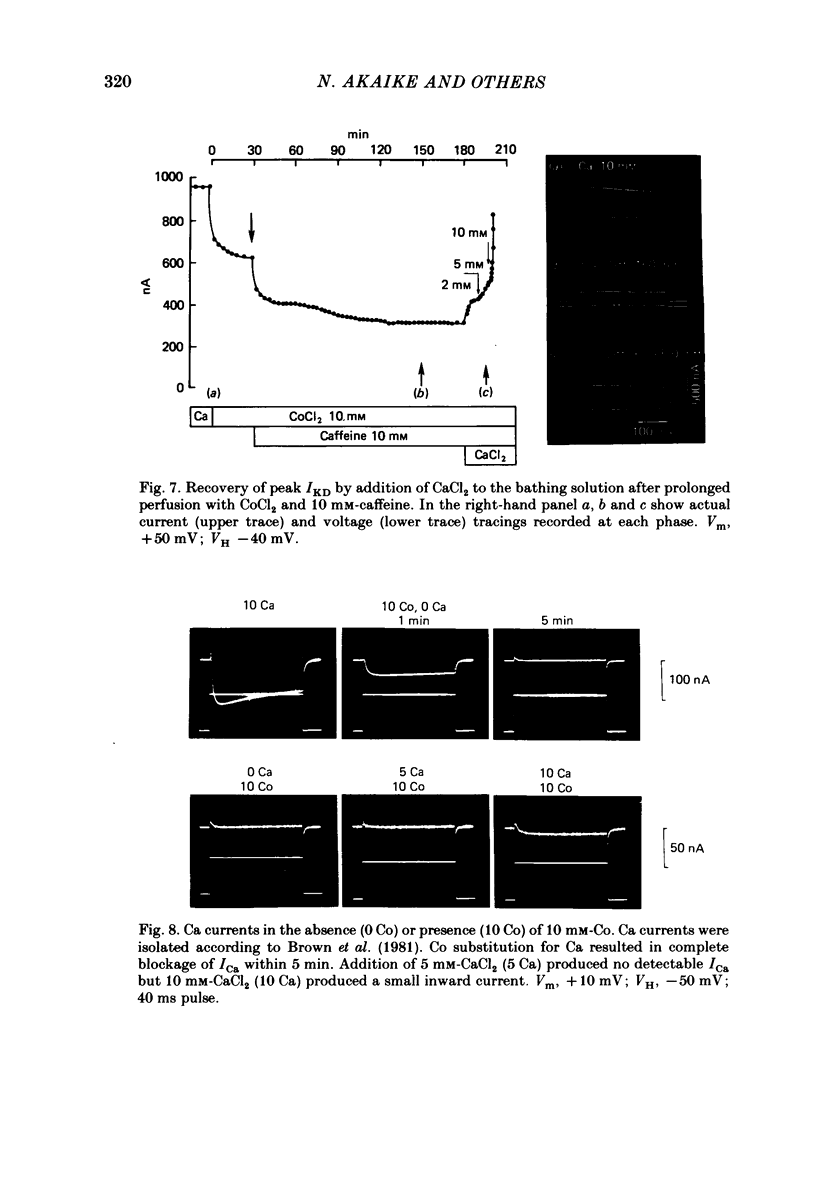
1. The effect of endogenous Ca on potential-dependent K current IKD, was examined in identifiable neurones of Helix aspersa. The suction pipette method of internal perfusion was used along with a combined voltage-clamp method in which the membrane potential was measured by a separate glass micro-electrode and the current was passed by the suction pipette. Activation of the potential-dependent A current, IA, was prevented by using holding potentials of -40 mV where IA is inactivated and by the addition of the A-current blocker 4-aminopyridine. Activation of K currents by transmembrane Ca current, IKCa, was suppressed by Co substitution for Ca ion extracellularly. 2. Under these conditions, IKD rose to a peak value and then subsided to a steady level. The current-voltage (I-V) relationship for peak IKD had an upward bump at about +50 mV that gave it an S-shape. The I-V curve for steady IKD rose continuously. Peak and steady IKD were reduced by perfusing with EGTA or F ions intracellularly. The EGTA effect occurred at intracellular Ca activity levels below 10(-7) M. Increases in the concentration of EGTAi at constant Cai had no additional effect; however, recovery experiments do not allow us to rule out some direct action of EGTA on IKD. 3. Prolonged extracellular perfusion with Co-substituted solutions also reduced IKD and the effects occurred more quickly when the solutions were made hypertonic or caffeine was added to them. The peak transient was abolished, and the small remaining steady IKD (about 5-10% of normal peak IKD) was blocked by tetraethylammonium. IKD could be restored by the temporary reintroduction of Ca in the extracellular solution. 4. The S-shape of the peak I-V relationship for IKD may be due to Ca released from an endogenous site by membrane depolarization. The reduction of steady and peak IKD to very low values by Ca chelators or prolonged perfusion with Ca-free solutions indicates that Cai is important for activation of these K channels. 5. Three cellular structures were identified in electron micrographs of freeze-fractured neurones that could be involved in potential-dependent endogenous Ca release. These were a restricted extracellularly space, an intracellular membrane system of endoplasmic reticulum that may be fused to the internal face of the plasma membrane (the subsurface cisterns of Henkart & Nelson, 1979), and intracellular vesicles that also may be fused to the plasma membrane.
Full text
PDF














Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahmed Z., Connor J. A. Measurement of calcium influx under voltage clamp in molluscan neurones using the metallochromic dye arsenazo III. J Physiol. 1979 Jan;286:61–82. doi: 10.1113/jphysiol.1979.sp012607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akaike N., Lee K. S., Brown A. M. The calcium current of Helix neuron. J Gen Physiol. 1978 May;71(5):509–531. doi: 10.1085/jgp.71.5.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aldrich R. W., Jr, Getting P. A., Thompson S. H. Inactivation of delayed outward current in molluscan neurone somata. J Physiol. 1979 Jun;291:507–530. doi: 10.1113/jphysiol.1979.sp012828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Branton D., Bullivant S., Gilula N. B., Karnovsky M. J., Moor H., Mühlethaler K., Northcote D. H., Packer L., Satir B., Satir P. Freeze-etching nomenclature. Science. 1975 Oct 3;190(4209):54–56. doi: 10.1126/science.1166299. [DOI] [PubMed] [Google Scholar]
- Brown A. M., Brodwick M. S., Eaton D. C. Intracellular calcium and extra-retinal photoreception of Aplysia Giant neurons. J Neurobiol. 1977 Jan;8(1):1–18. doi: 10.1002/neu.480080102. [DOI] [PubMed] [Google Scholar]
- Brown A. M., Morimoto K., Tsuda Y., wilson D. L. Calcium current-dependent and voltage-dependent inactivation of calcium channels in Helix aspersa. J Physiol. 1981 Nov;320:193–218. doi: 10.1113/jphysiol.1981.sp013944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connor J. A., Stevens C. F. Voltage clamp studies of a transient outward membrane current in gastropod neural somata. J Physiol. 1971 Feb;213(1):21–30. doi: 10.1113/jphysiol.1971.sp009365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahl G., Isenberg G. Decoupling of heart muscle cells: correlation with increased cytoplasmic calcium activity and with changes of nexus ultrastructure. J Membr Biol. 1980 Mar 31;53(1):63–75. doi: 10.1007/BF01871173. [DOI] [PubMed] [Google Scholar]
- Endo M. Calcium release from the sarcoplasmic reticulum. Physiol Rev. 1977 Jan;57(1):71–108. doi: 10.1152/physrev.1977.57.1.71. [DOI] [PubMed] [Google Scholar]
- Gorman A. L., Thomas M. V. Intracellular calcium accumulation during depolarization in a molluscan neurone. J Physiol. 1980 Nov;308:259–285. doi: 10.1113/jphysiol.1980.sp013471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henkart M. P., Nelson P. G. Evidence for an intracellular calcium store releasable by surface stimuli ifibroblasts (L cells). J Gen Physiol. 1979 May;73(5):655–673. doi: 10.1085/jgp.73.5.655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henkart M., Landis D. M., Reese T. S. Similarity of junctions between plasma membranes and endoplasmic reticulum in muscle and neurons. J Cell Biol. 1976 Aug;70(2 Pt 1):338–347. doi: 10.1083/jcb.70.2.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heyer C. B., Lux H. D. Control of the delayed outward potassium currents in bursting pace-maker neurones of the snail, Helix pomatia. J Physiol. 1976 Nov;262(2):349–382. doi: 10.1113/jphysiol.1976.sp011599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue I. Separation of the action potential into a Na-channel spike and a K-channel spike by tetrodotoxin and by tetraethylammonium ion in squid giant axons internally perfused with dilute Na-salt solutions. J Gen Physiol. 1980 Sep;76(3):337–354. doi: 10.1085/jgp.76.3.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerkut G. A., Lambert J. D., Gayton R. J., Loker J. E., Walker R. J. Mapping of nerve cells in the suboesophageal ganglia of Helix aspersa. Comp Biochem Physiol A Comp Physiol. 1975 Jan 1;50(1A):1–25. doi: 10.1016/s0010-406x(75)80194-0. [DOI] [PubMed] [Google Scholar]
- Kostyuk P. G., Krishtal O. A. Effects of calcium and calcium-chelating agents on the inward and outward current in the membrane of mollusc neurones. J Physiol. 1977 Sep;270(3):569–580. doi: 10.1113/jphysiol.1977.sp011969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K. S., Akaike N., Brown A. M. The suction pipette method for internal perfusion and voltage clamp of small excitable cells. J Neurosci Methods. 1980 Feb;2(1):51–78. doi: 10.1016/0165-0270(80)90045-x. [DOI] [PubMed] [Google Scholar]
- Lux H. D., Neher E., Marty A. Single channel activity associated with the calcium dependent outward current in Helix pomatia. Pflugers Arch. 1981 Mar;389(3):293–295. doi: 10.1007/BF00584792. [DOI] [PubMed] [Google Scholar]
- Marty A. Ca-dependent K channels with large unitary conductance in chromaffin cell membranes. Nature. 1981 Jun 11;291(5815):497–500. doi: 10.1038/291497a0. [DOI] [PubMed] [Google Scholar]
- Meech R. W. Calcium-dependent potassium activation in nervous tissues. Annu Rev Biophys Bioeng. 1978;7:1–18. doi: 10.1146/annurev.bb.07.060178.000245. [DOI] [PubMed] [Google Scholar]
- Meech R. W., Standen N. B. Potassium activation in Helix aspersa neurones under voltage clamp: a component mediated by calcium influx. J Physiol. 1975 Jul;249(2):211–239. doi: 10.1113/jphysiol.1975.sp011012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meech R. W., Thomas R. C. Effect of measured calcium chloride injections on the membrane potential and internal pH of snail neurones. J Physiol. 1980 Jan;298:111–129. doi: 10.1113/jphysiol.1980.sp013070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meves H., Vogel W. Calcium inward currents in internally perfused giant axons. J Physiol. 1973 Nov;235(1):225–265. doi: 10.1113/jphysiol.1973.sp010386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miledi R., Parker I., Schalow G. Measurement of changes in intracellular calcium in frog skeletal muscle fibres using arsenazo III [proceedings]. J Physiol. 1977 Jul;269(1):11P–13P. [PubMed] [Google Scholar]
- Neher E. Two fast transient current components during voltage clamp on snail neurons. J Gen Physiol. 1971 Jul;58(1):36–53. doi: 10.1085/jgp.58.1.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page E., Upshaw-Earley J. Volume changes in sarcoplasmic reticulum of rat hearts perfused with hypertonic solutions. Circ Res. 1977 Apr;40(4):355–366. doi: 10.1161/01.res.40.4.355. [DOI] [PubMed] [Google Scholar]
- Pallotta B. S., Magleby K. L., Barrett J. N. Single channel recordings of Ca2+-activated K+ currents in rat muscle cell culture. Nature. 1981 Oct 8;293(5832):471–474. doi: 10.1038/293471a0. [DOI] [PubMed] [Google Scholar]
- ROSENBLUTH J. Subsurface cisterns and their relationship to the neuronal plasma membrane. J Cell Biol. 1962 Jun;13:405–421. doi: 10.1083/jcb.13.3.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson S. H. Three pharmacologically distinct potassium channels in molluscan neurones. J Physiol. 1977 Feb;265(2):465–488. doi: 10.1113/jphysiol.1977.sp011725. [DOI] [PMC free article] [PubMed] [Google Scholar]




