Abstract
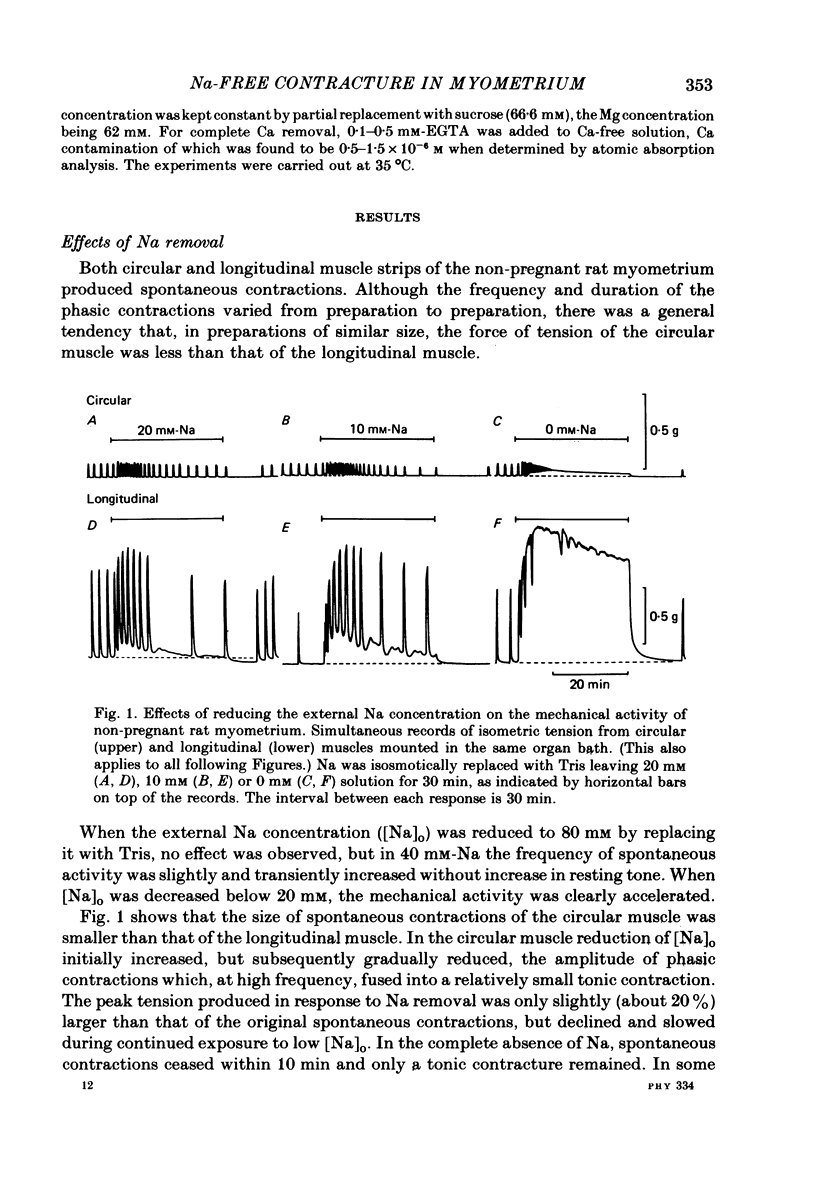
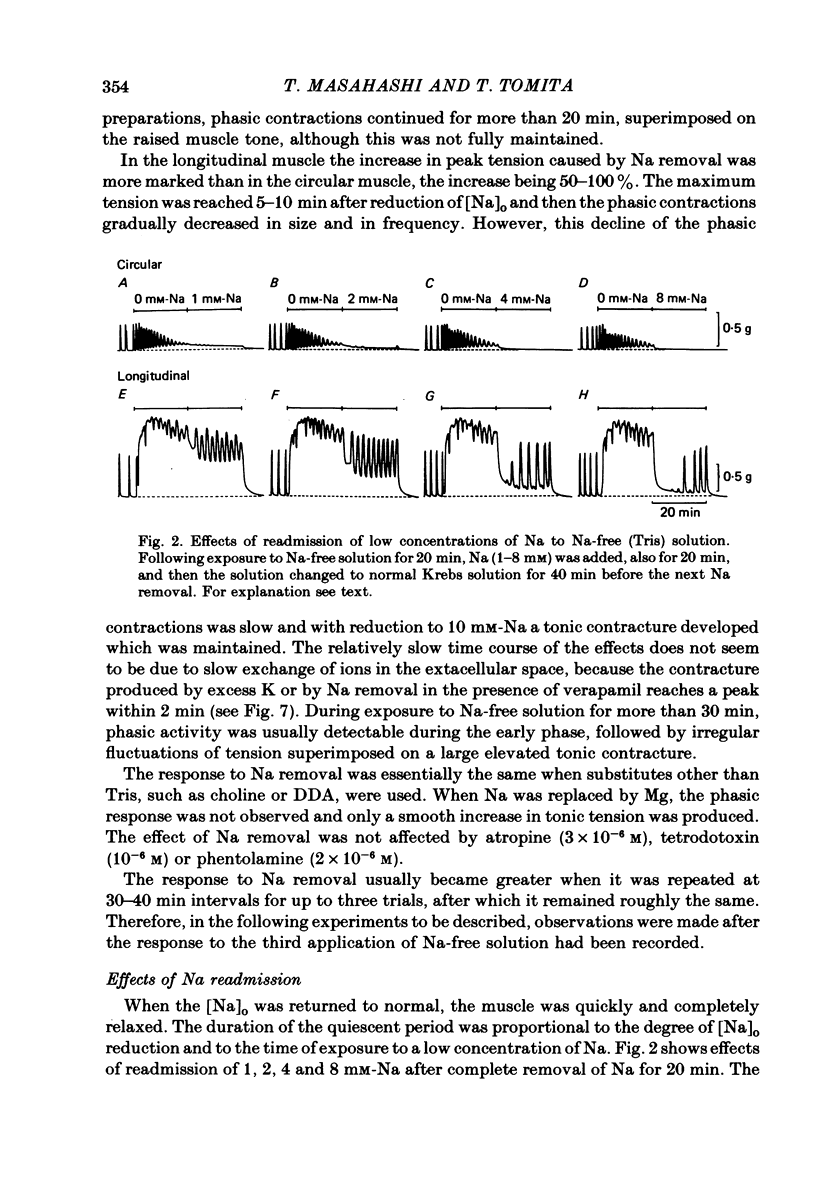
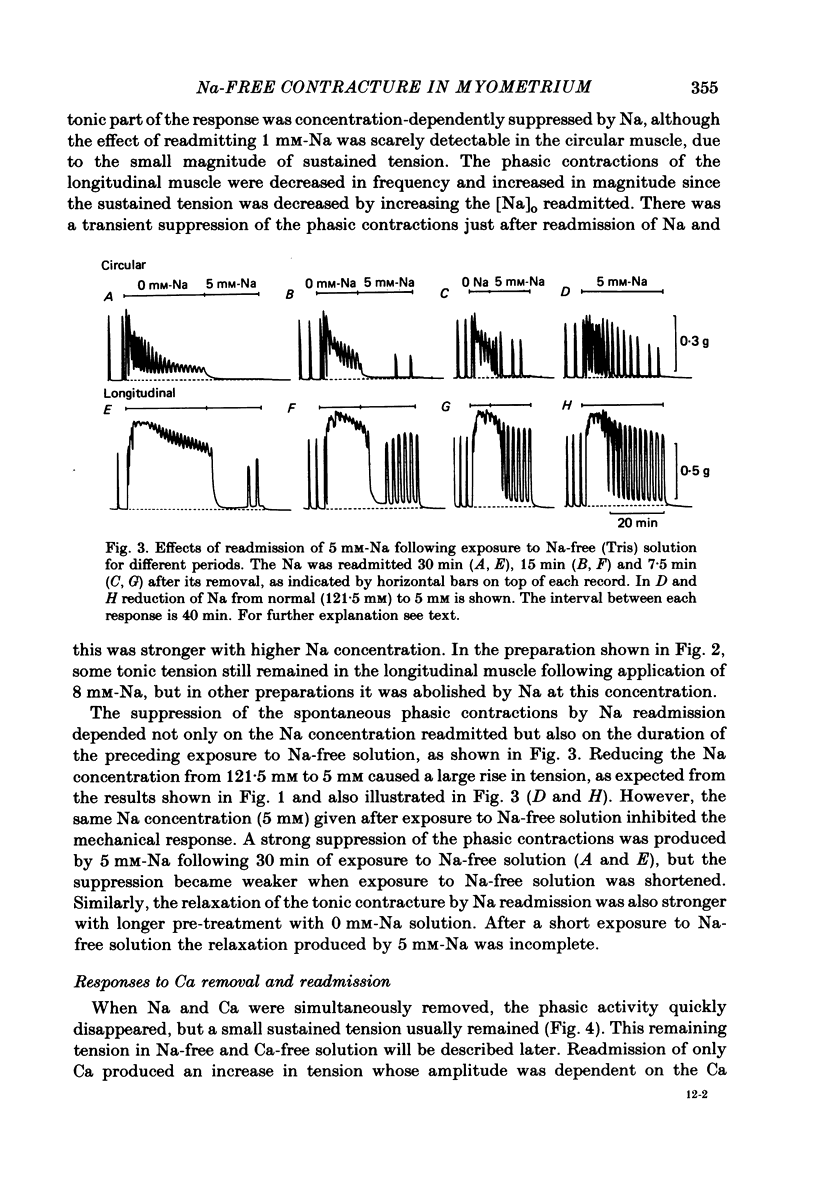
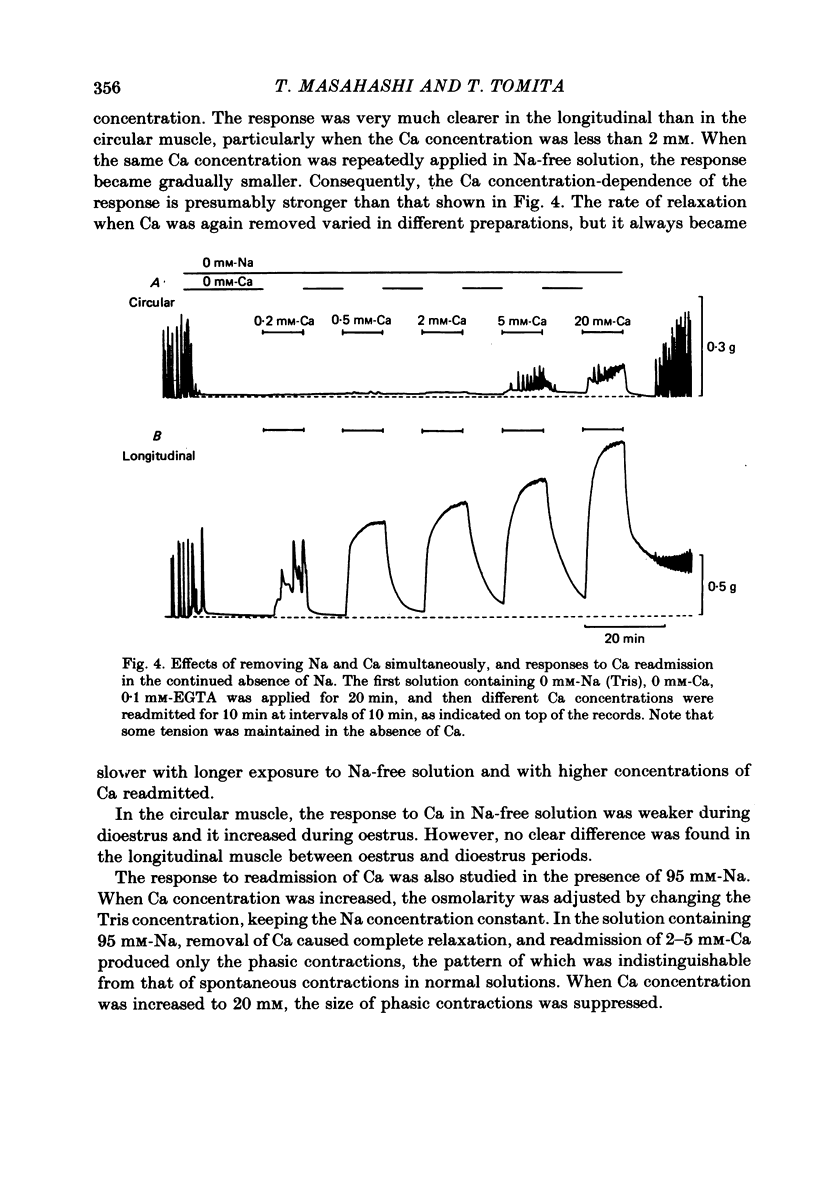
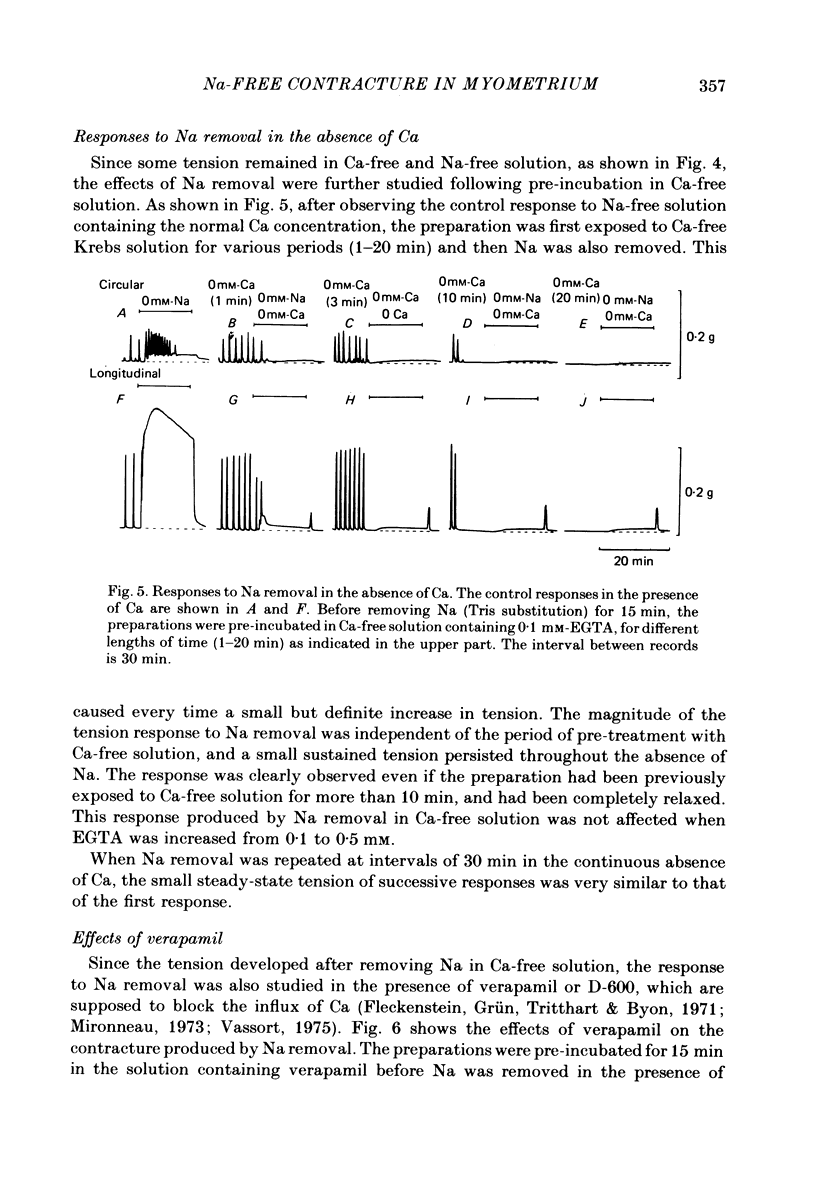
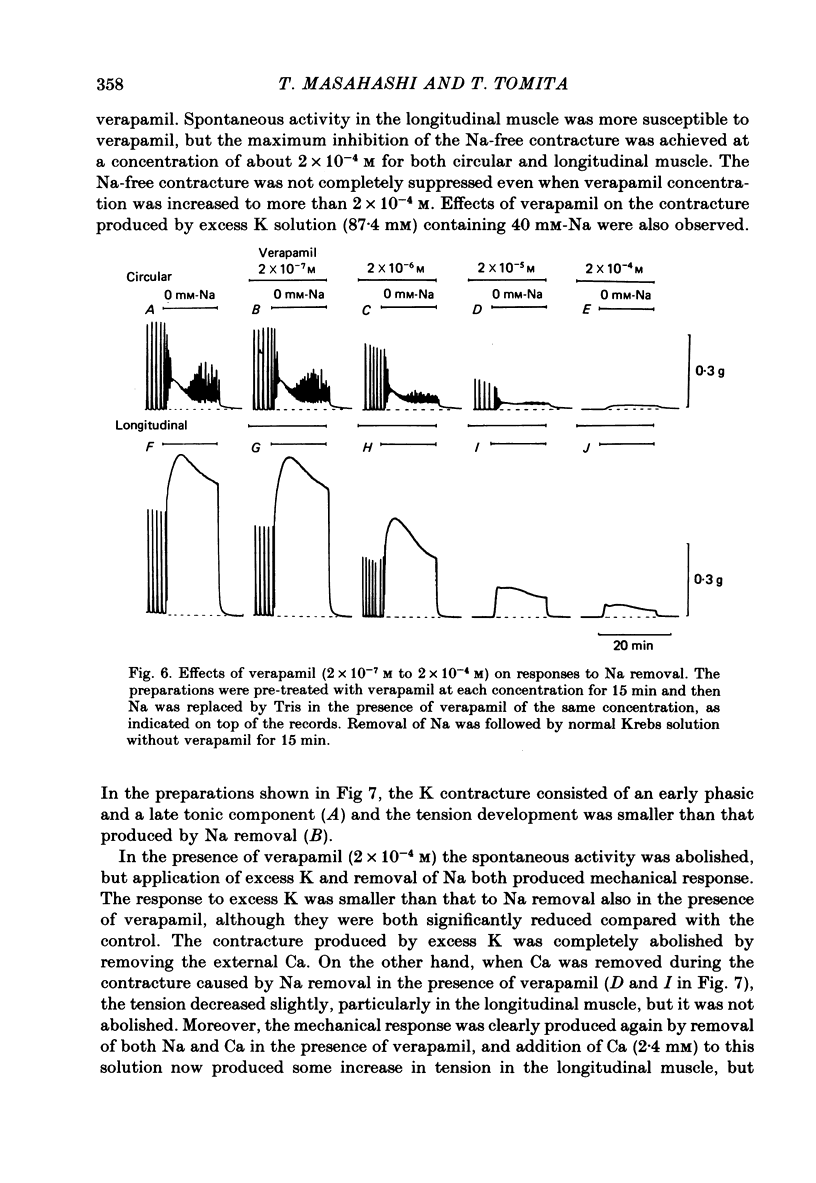
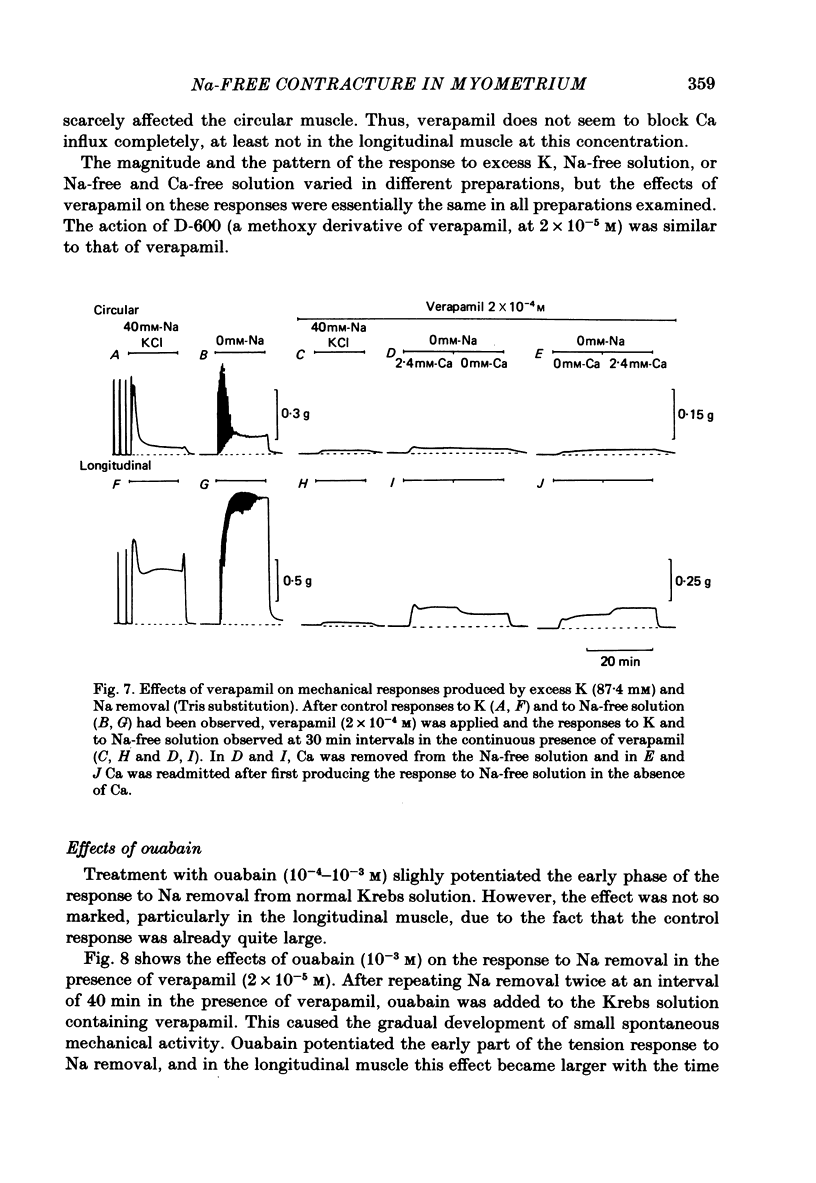
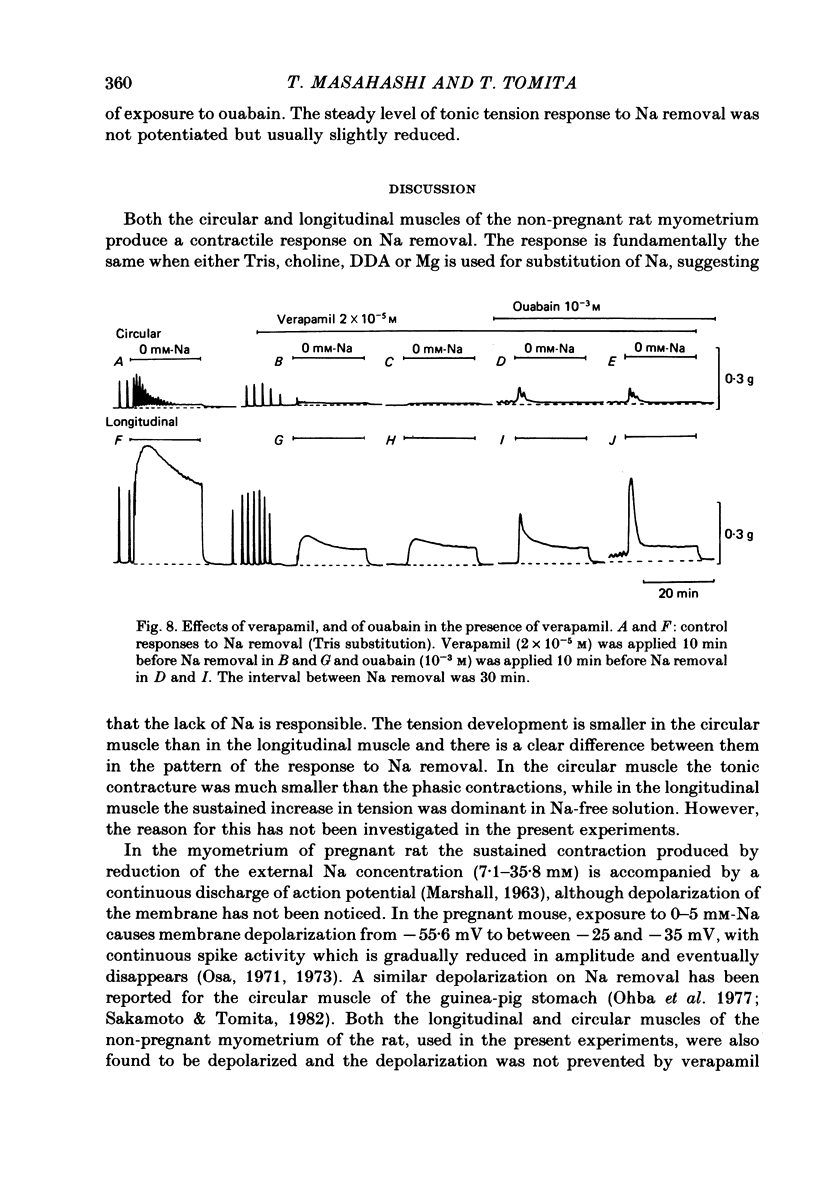
1. Mechanical responses to Na removal were investigated in the circular and longitudinal muscles of the non-pregnant rat myometrium at 35 degrees C. In both muscles, reduction of the external Na concentration to less than 20 mM produced an initial acceleration of phasic contractions and a sustained tonic contracture. No difference was found with different Na substitutes (Tris-hydroxymethyl aminomethane, choline, dimethyl diethanol ammonium). However, when Mg was substituted for Na, only the tonic contracture was produced without the phasic contractions. 2. Readmission of 5-10 mM-Na, after exposure to Na-free solution, relaxed the contracture produced by Na removal. The degree of relaxation was dependent on the Na concentration readmitted and on the period of pre-treatment with Na-free solution, being stronger with longer pre-treatment. 3. In the presence of Na, excess Ca failed to increase the muscle tone. In the absence of Na, the tension development was closely related to the external Ca concentration up to 20 mM. In the absence of both Ca and Na, some tension remained. Even after pre-treatment with Ca-free solution containing 0.1-0.5 mM EGTA, removal of Na caused some mechanical response. A similar small tension development was observed when Na removal was repeated during prolonged absence of external Ca for more than 3 h. 4. Verapamil (2 X 10(-4) M) markedly suppressed the response to Na removal, but it did not block it, either in the presence or in the absence of Ca. Ouabain (10(-3) M) in the presence of verapamil potentiated the early phasic component of the response to Na removal, but the tonic component was little affected or even slightly reduced. 5. The results indicate that there are three components in the mechanical response to Na removal: the phasic and tonic components, which are highly Ca-dependent, and the third small tonic component, which is independent of external Ca. Most of the phasic and tonic responses seem to be due to an increase in Ca permeability, but this may be secondary to membrane depolarization. A Na-Ca exchange mechanism is also considered to contribute to the transient phase of the response to Na removal and to Na readmission.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baker P. F., Blaustein M. P., Hodgkin A. L., Steinhardt R. A. The influence of calcium on sodium efflux in squid axons. J Physiol. 1969 Feb;200(2):431–458. doi: 10.1113/jphysiol.1969.sp008702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brading A. F., Burnett M., Sneddon P. The effect of sodium removal on the contractile response of the guinea-pig taenia coli to carbachol. J Physiol. 1980 Sep;306:411–429. doi: 10.1113/jphysiol.1980.sp013404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleckenstein A., Grün G., Tritthart H., Byon K. Uterus-Relaxation durch hochaktive Ca plus,plus-antagonistische Hemmstoffe der elektro-mechanischen Koppelung wie Isoptin (Verapamil, Iproveratril), Substanz D 600 und Segontin (Prenylamin). Versuche am isolierten Uterus virgineller Ratten. Klin Wochenschr. 1971 Jan;49(1):32–41. doi: 10.1007/BF01494064. [DOI] [PubMed] [Google Scholar]
- Ma T. S., Bose D. Sodium in smooth muscle relaxation. Am J Physiol. 1977 Jan;232(1):C59–C66. doi: 10.1152/ajpcell.1977.232.1.C59. [DOI] [PubMed] [Google Scholar]
- Mironneau J. Excitation-contraction coupling in voltage clamped uterine smooth muscle. J Physiol. 1973 Aug;233(1):127–141. doi: 10.1113/jphysiol.1973.sp010301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohba M., Sakamoto Y., Tomita T. Effects of sodium, potassium and calcium ions on the slow wave in the circular muscle of the guinea-pig stomach. J Physiol. 1977 May;267(1):167–180. doi: 10.1113/jphysiol.1977.sp011806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osa T. Effect of removing the external sodium on the electrical and mechanical activities of the pregnant mouse myometrium. Jpn J Physiol. 1971 Dec;21(6):607–625. doi: 10.2170/jjphysiol.21.607. [DOI] [PubMed] [Google Scholar]
- Osa T. The effects of sodium, calcium and manganese on the electrical and mechanical activities of the myometrial smooth muscle of pregnant mice. Jpn J Physiol. 1973 Apr;23(2):113–133. doi: 10.2170/jjphysiol.23.113. [DOI] [PubMed] [Google Scholar]
- Reuter H., Seitz N. The dependence of calcium efflux from cardiac muscle on temperature and external ion composition. J Physiol. 1968 Mar;195(2):451–470. doi: 10.1113/jphysiol.1968.sp008467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakamoto Y., Tomita T. Depolarization produced by sodium removal in the circular muscle of the guinea-pig stomach. J Physiol. 1982 May;326:329–339. doi: 10.1113/jphysiol.1982.sp014196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Breemen C., Aaronson P., Loutzenhiser R. Sodium-calcium interactions in mammalian smooth muscle. Pharmacol Rev. 1978 Jun;30(2):167–208. [PubMed] [Google Scholar]
- Vassort G. Voltage-clamp analysis of transmembrane ionic currents in guinea-pig myometrium: evidence for an initial potassium activation triggered by calcium influx. J Physiol. 1975 Nov;252(3):713–734. doi: 10.1113/jphysiol.1975.sp011167. [DOI] [PMC free article] [PubMed] [Google Scholar]


