Abstract
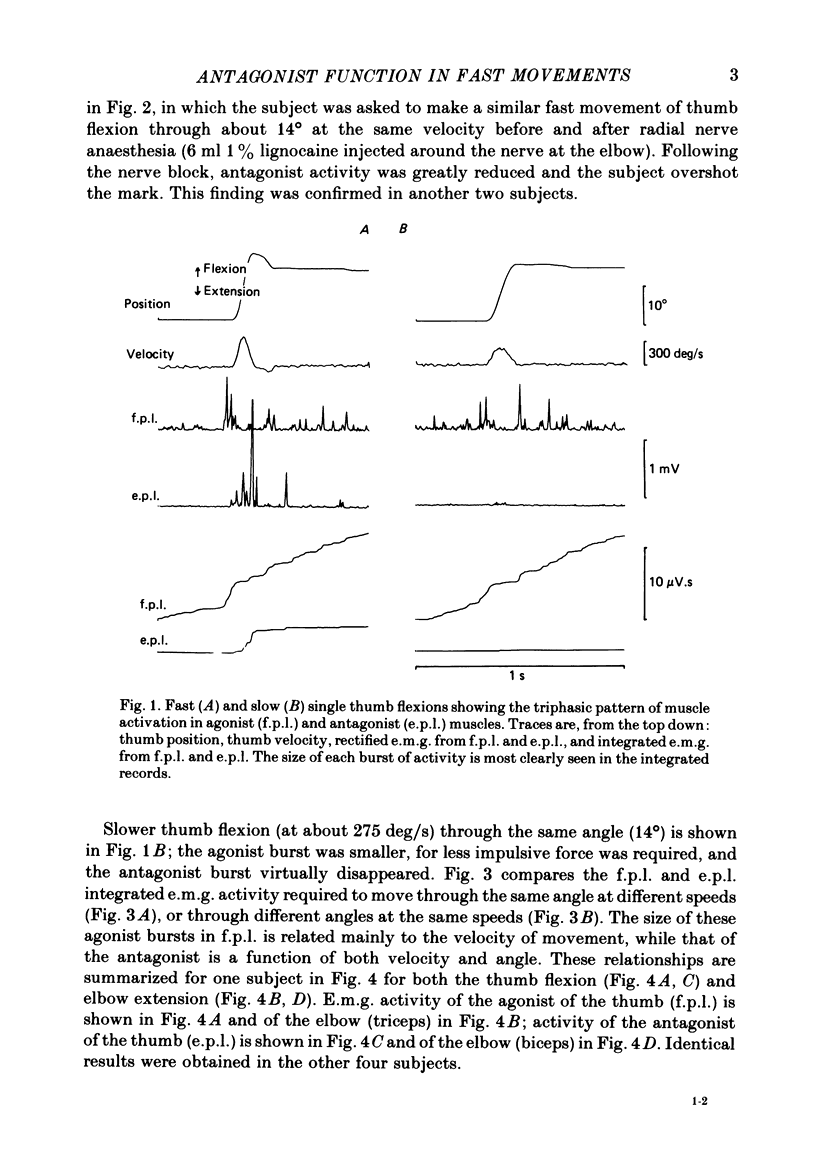
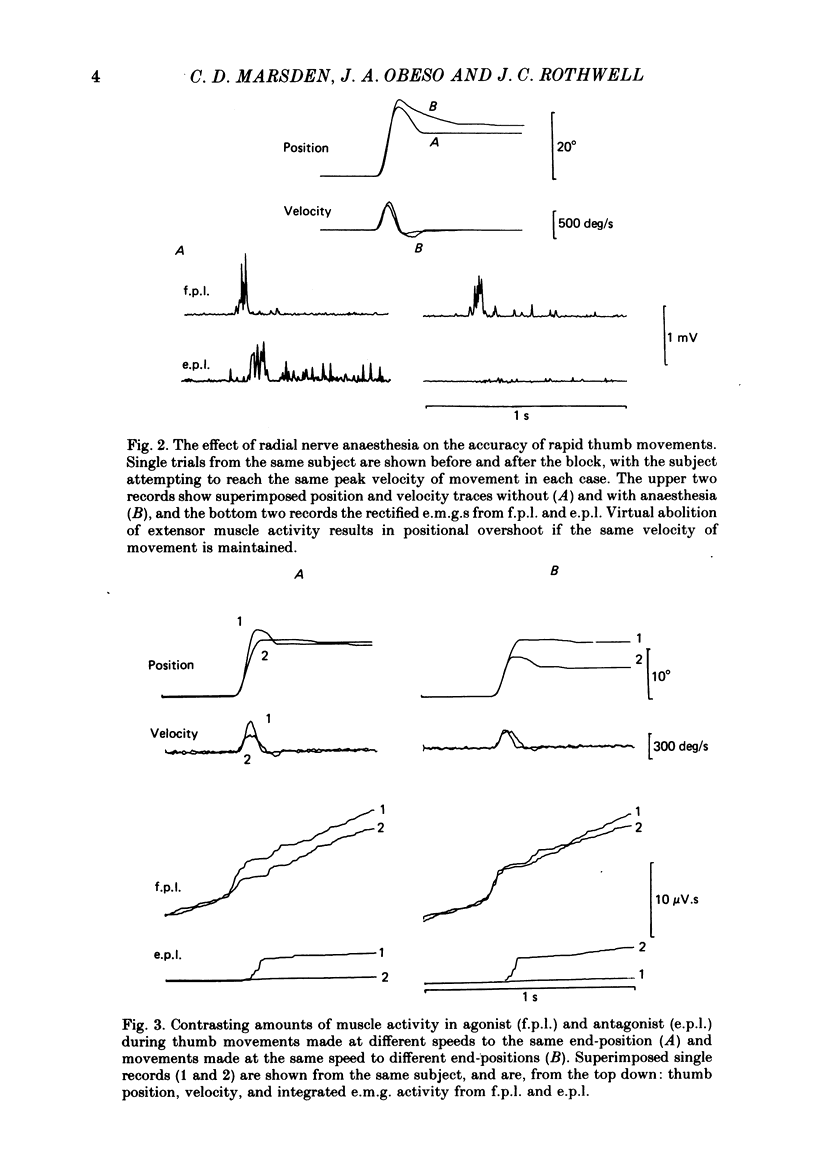
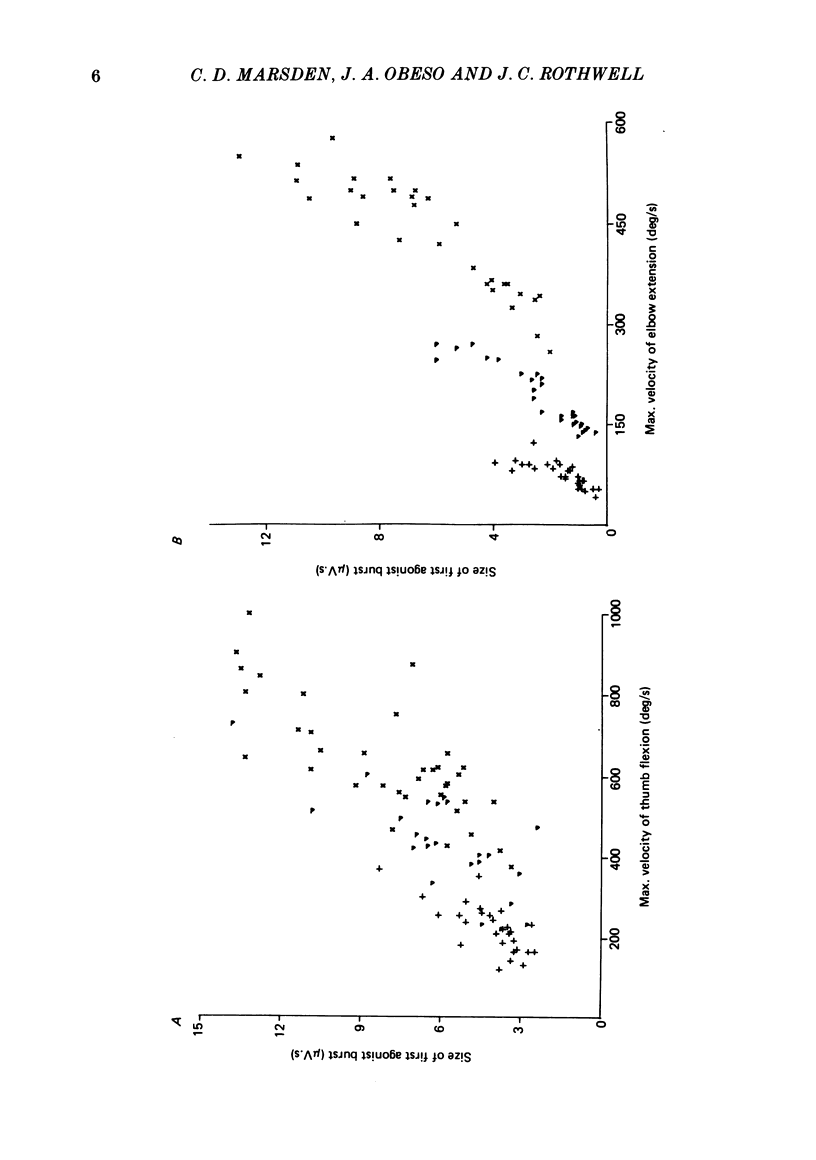
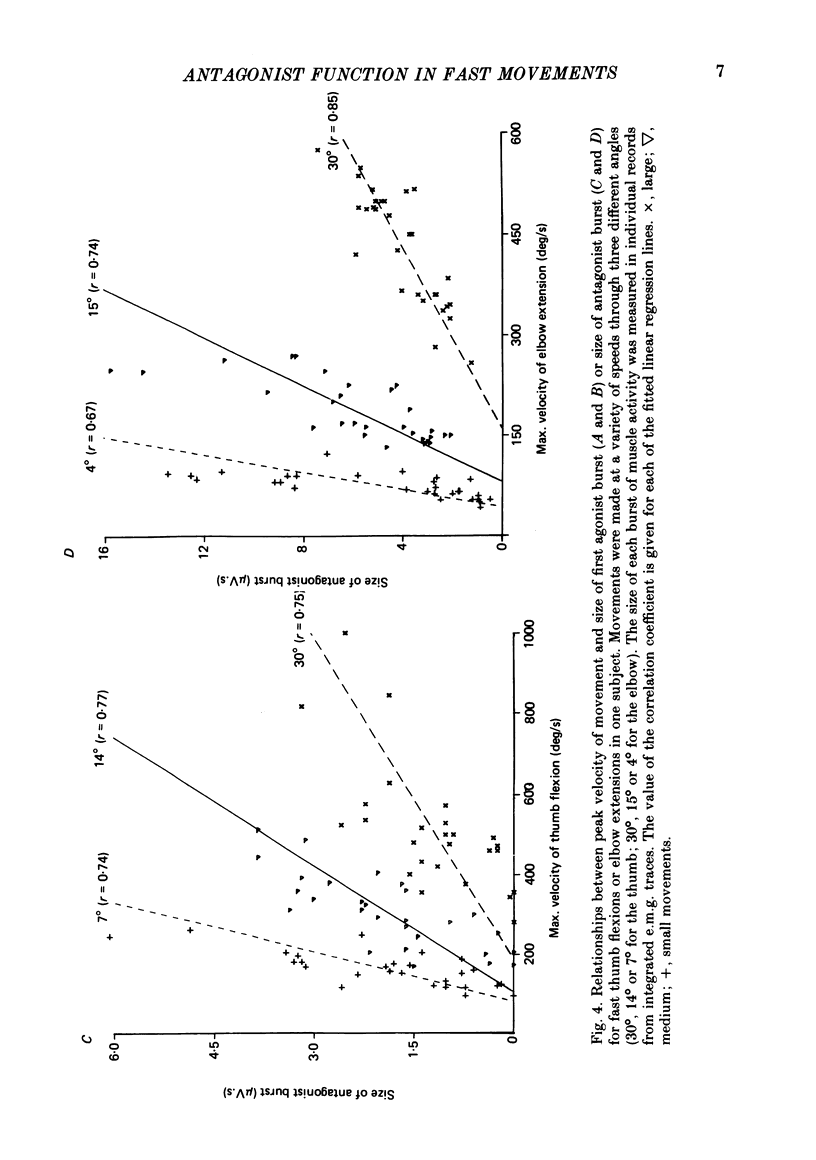
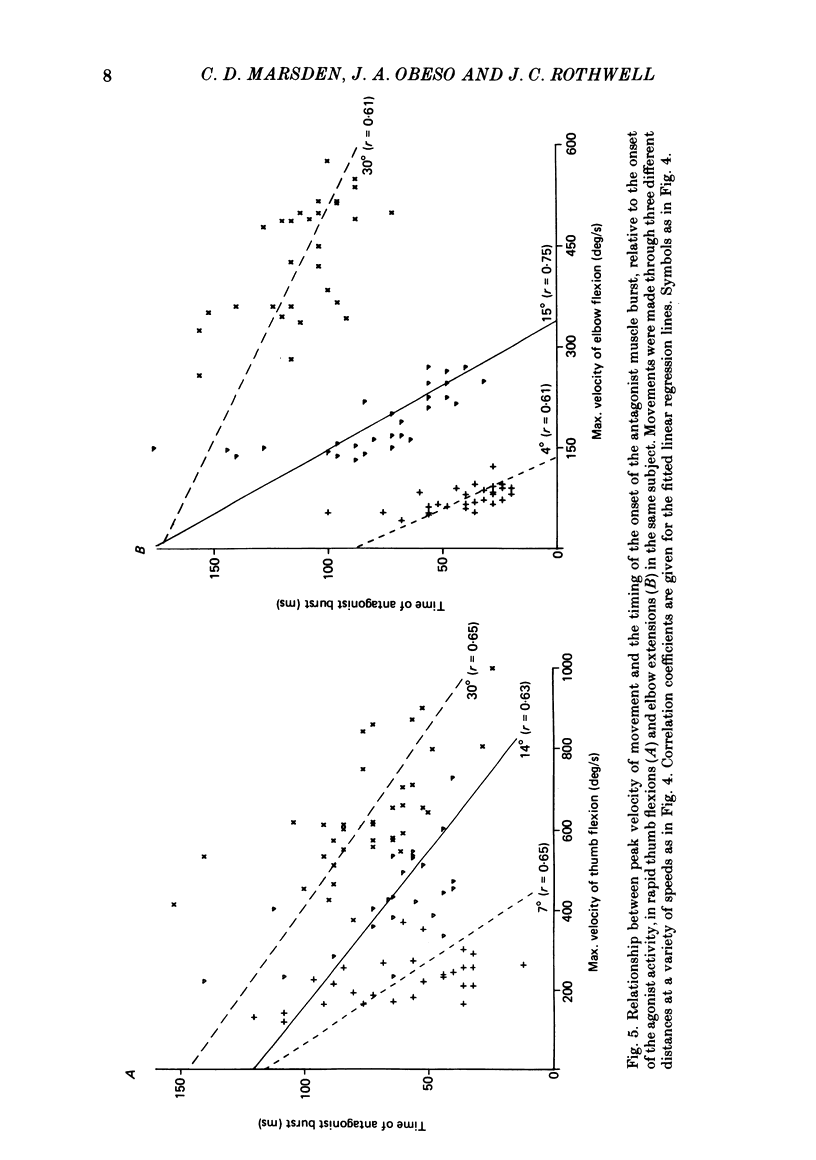
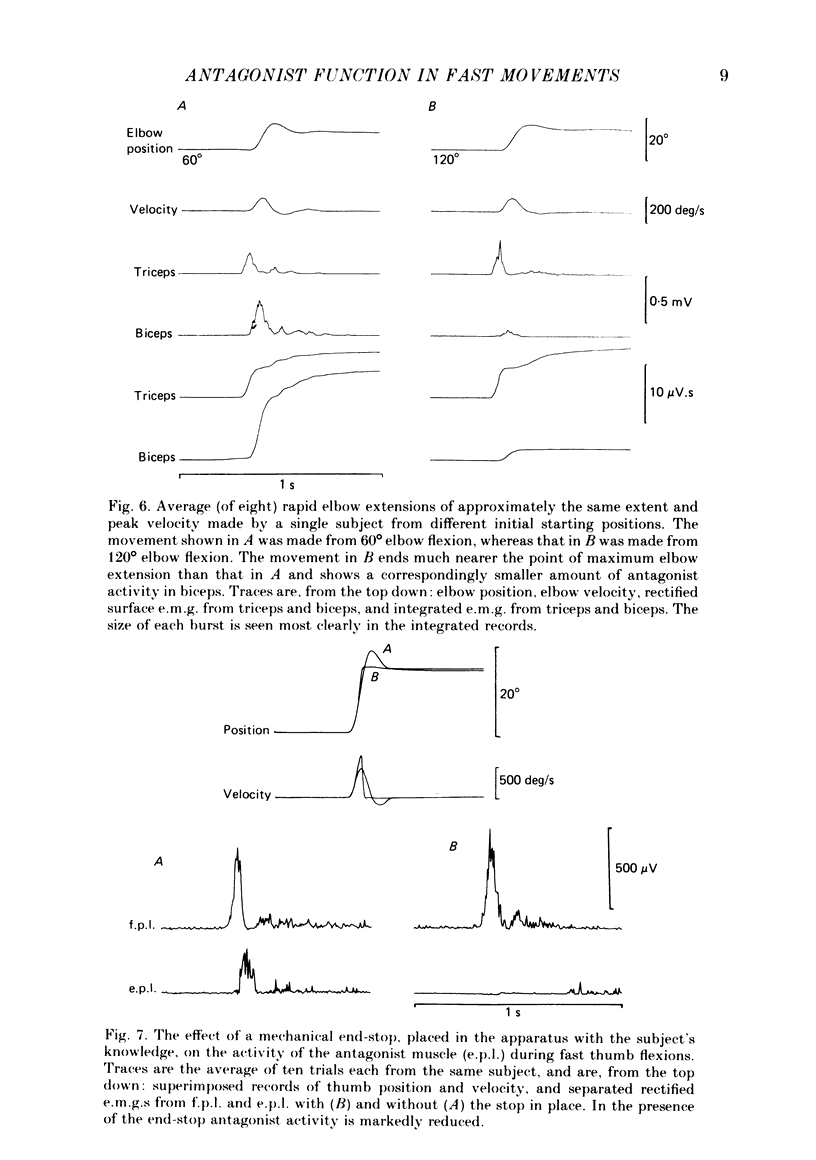
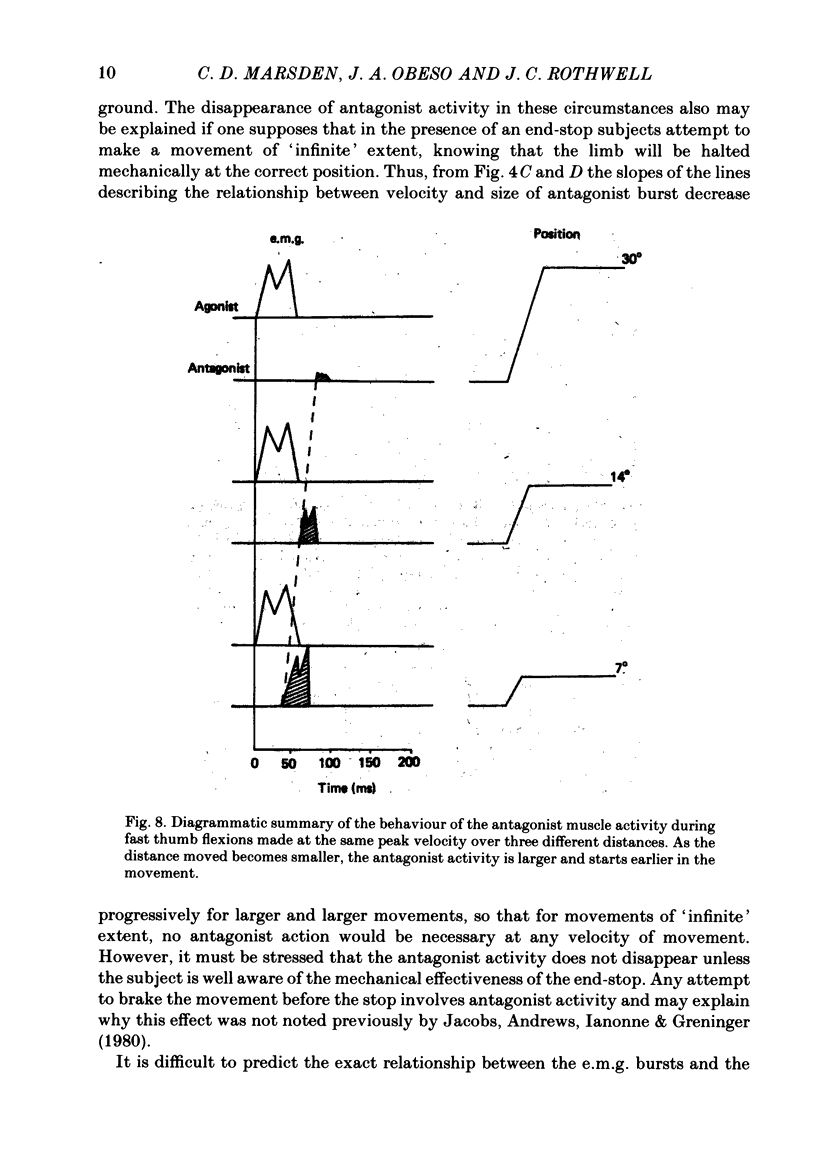
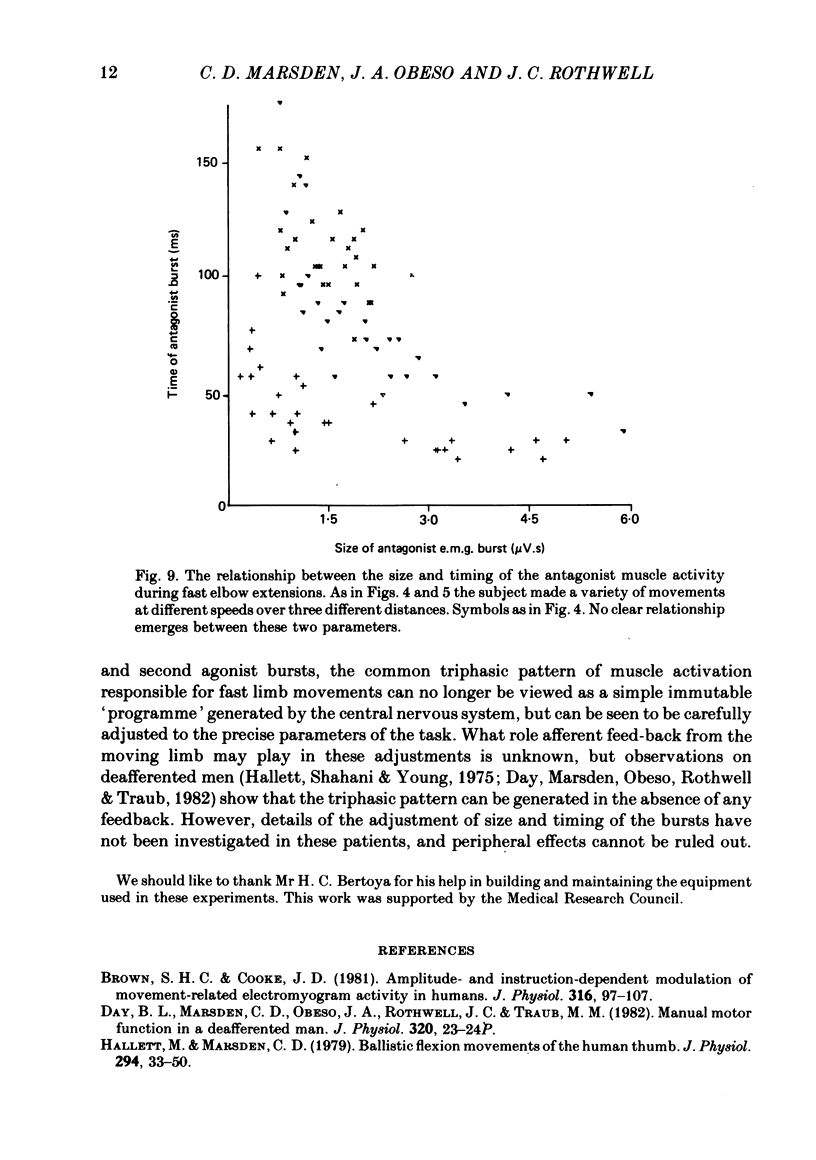
We have examined fast flexion movements of the human thumb and fast extension movements of the elbow over three different distances at a variety of speeds in order to elucidate the function of the antagonist muscle in these circumstances. All movements were of such a velocity that they showed the typical bi- or triphasic pattern of muscle activation in agonist and antagonist. Slower movements, with continuous agonist activity, were not analysed. For movements made through the same angle at different velocities, there was a linear relationship between the amount of antagonist activity needed to halt the movement and the peak velocity. However, the slope of this relationship was a function of the distance moved. Movements made through large angles showed less antagonist activity than those made through small angles at the same speed. The timing of the antagonist activity also changed as a function of both distance and speed. Fast, small movements showed earlier onset of antagonist activity than slow, large ones. Movements which were halted mechanically with the subject's prior knowledge had little or no antagonist activity, since it was no longer necessary in these conditions. The complexity of these relations indicates that the triphasic pattern of muscle activity underlying these movements can no longer be regarded as a simple immutable 'programme'. The size and timing of the bursts of muscle activity are subtly adjusted to the precise nature of the task.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brown S. H., Cooke J. D. Amplitude- and instruction-dependent modulation of movement-related electromyogram activity in humans. J Physiol. 1981 Jul;316:97–107. doi: 10.1113/jphysiol.1981.sp013775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallett M., Marsden C. D. Ballistic flexion movements of the human thumb. J Physiol. 1979 Sep;294:33–50. doi: 10.1113/jphysiol.1979.sp012913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallett M., Shahani B. T., Young R. R. EMG analysis of stereotyped voluntary movements in man. J Neurol Neurosurg Psychiatry. 1975 Dec;38(12):1154–1162. doi: 10.1136/jnnp.38.12.1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs M. B., Andrews L. T., Iannone A., Greninger L. Antagonist EMG temporal patterns during rapid voluntary movement. Neurology. 1980 Jan;30(1):36–41. doi: 10.1212/wnl.30.1.36. [DOI] [PubMed] [Google Scholar]
- Lestienne F. Effects of inertial load and velocity on the braking process of voluntary limb movements. Exp Brain Res. 1979 May 2;35(3):407–418. doi: 10.1007/BF00236760. [DOI] [PubMed] [Google Scholar]


