Abstract
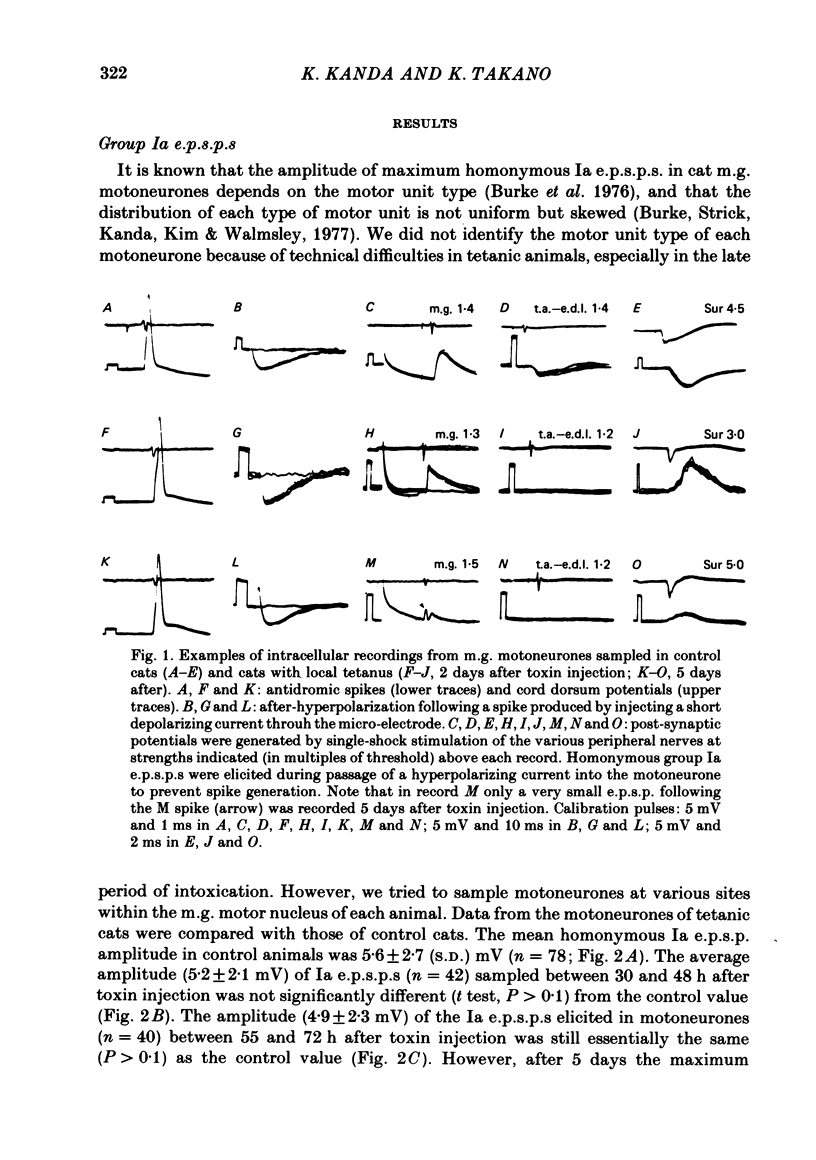
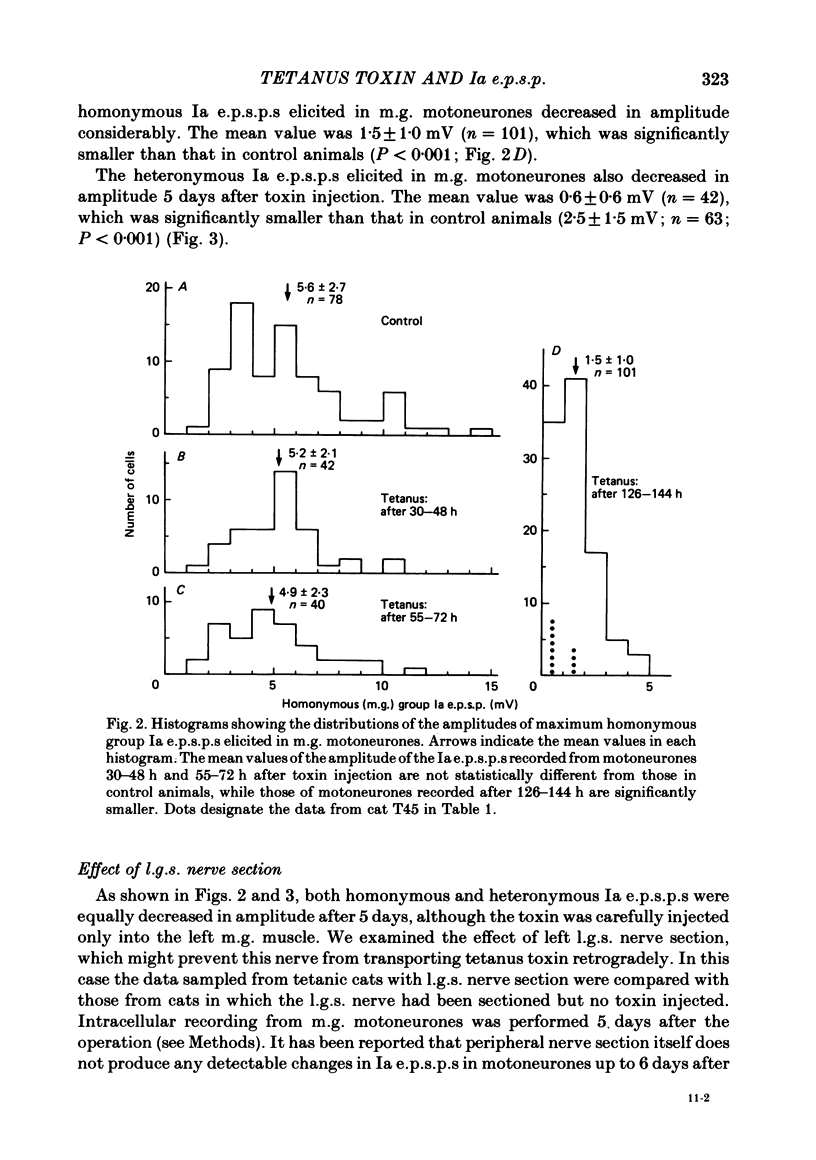
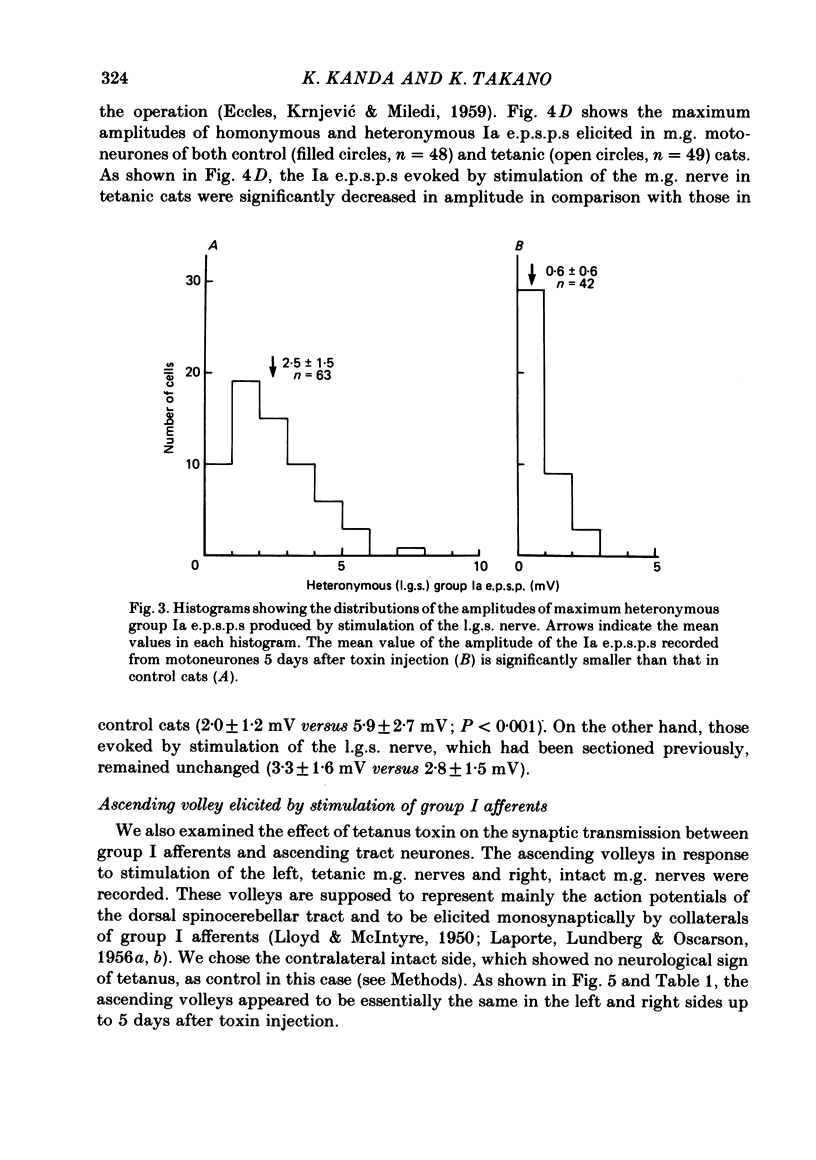
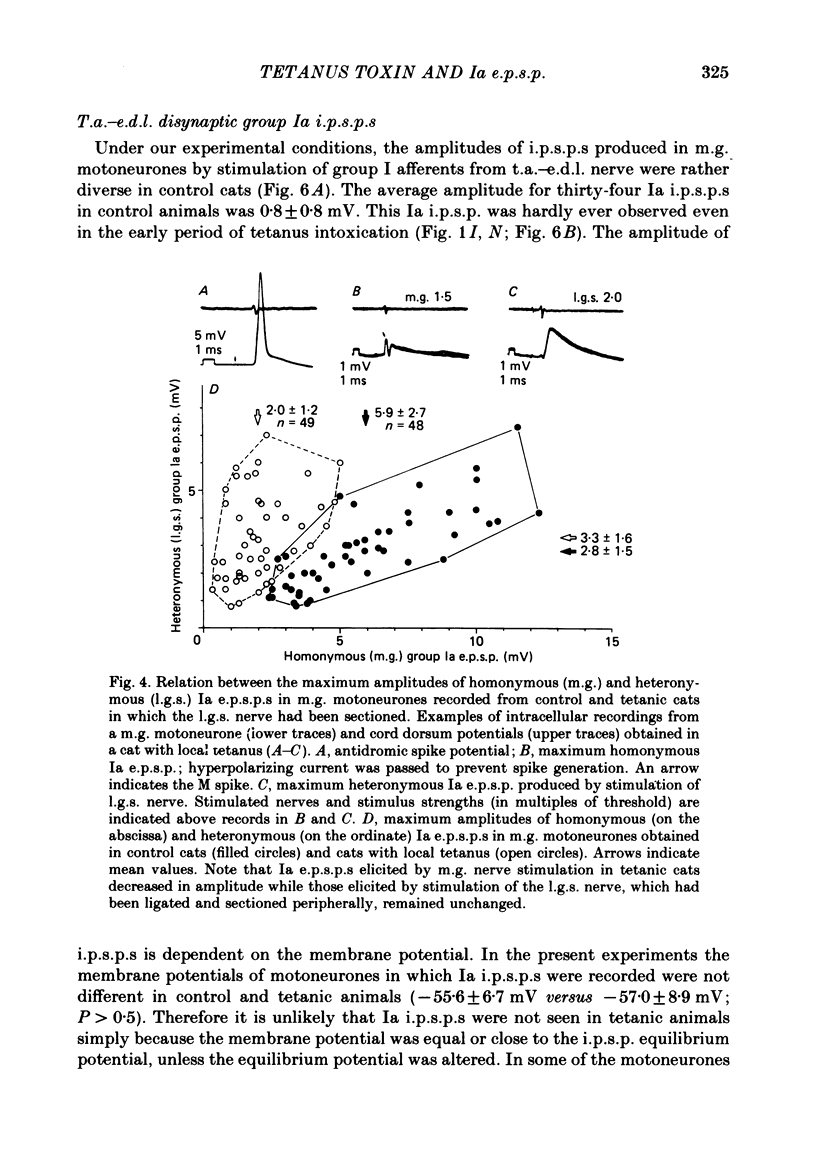
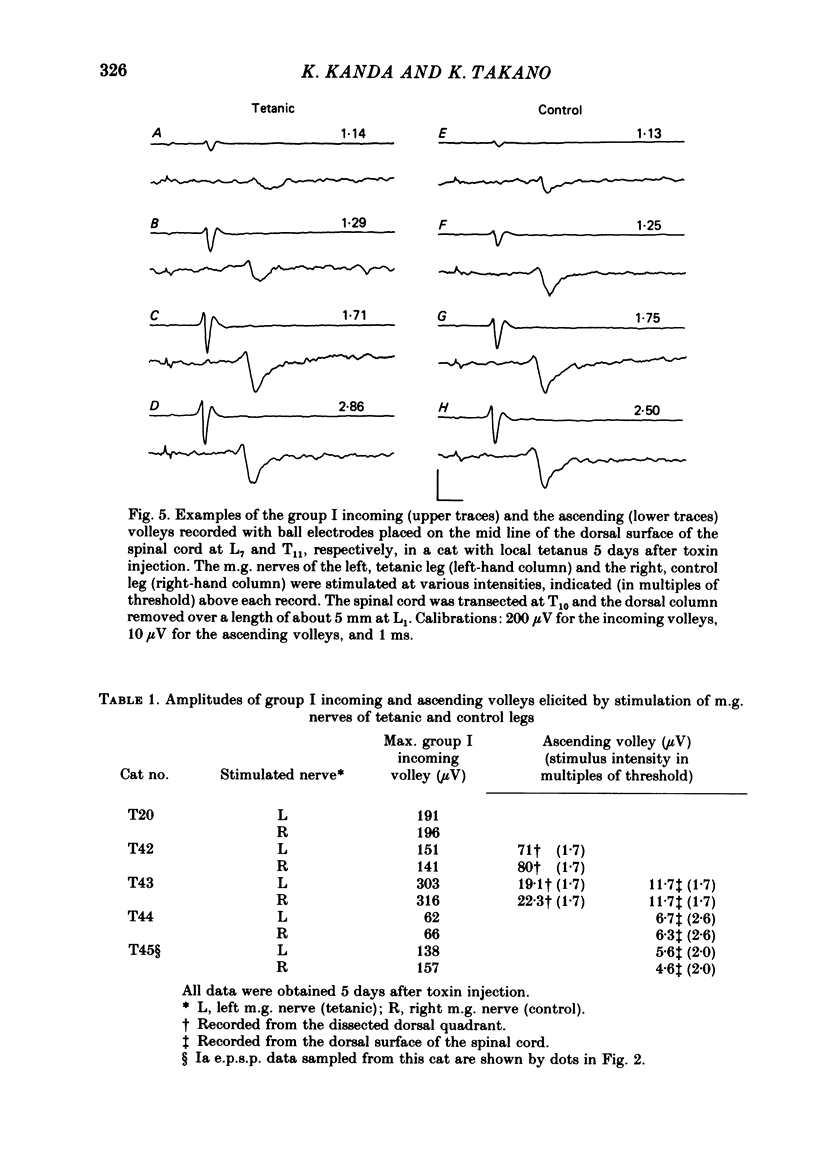
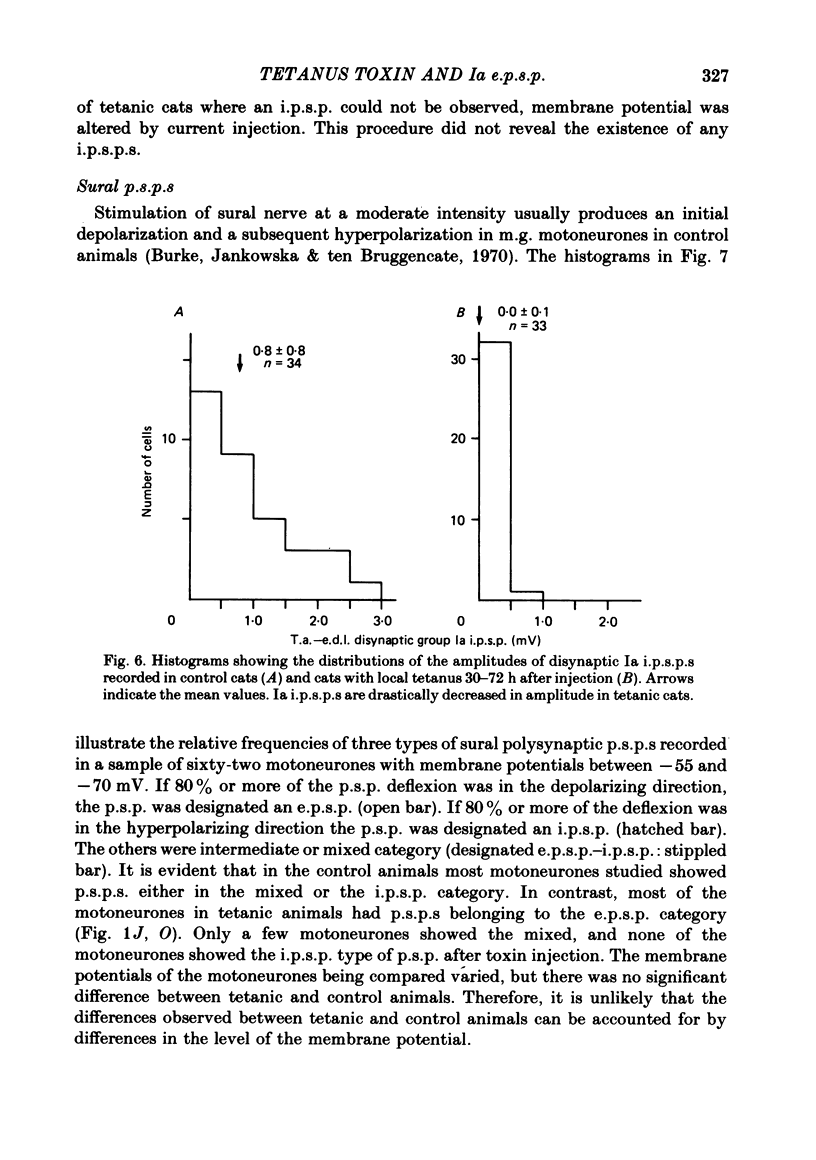
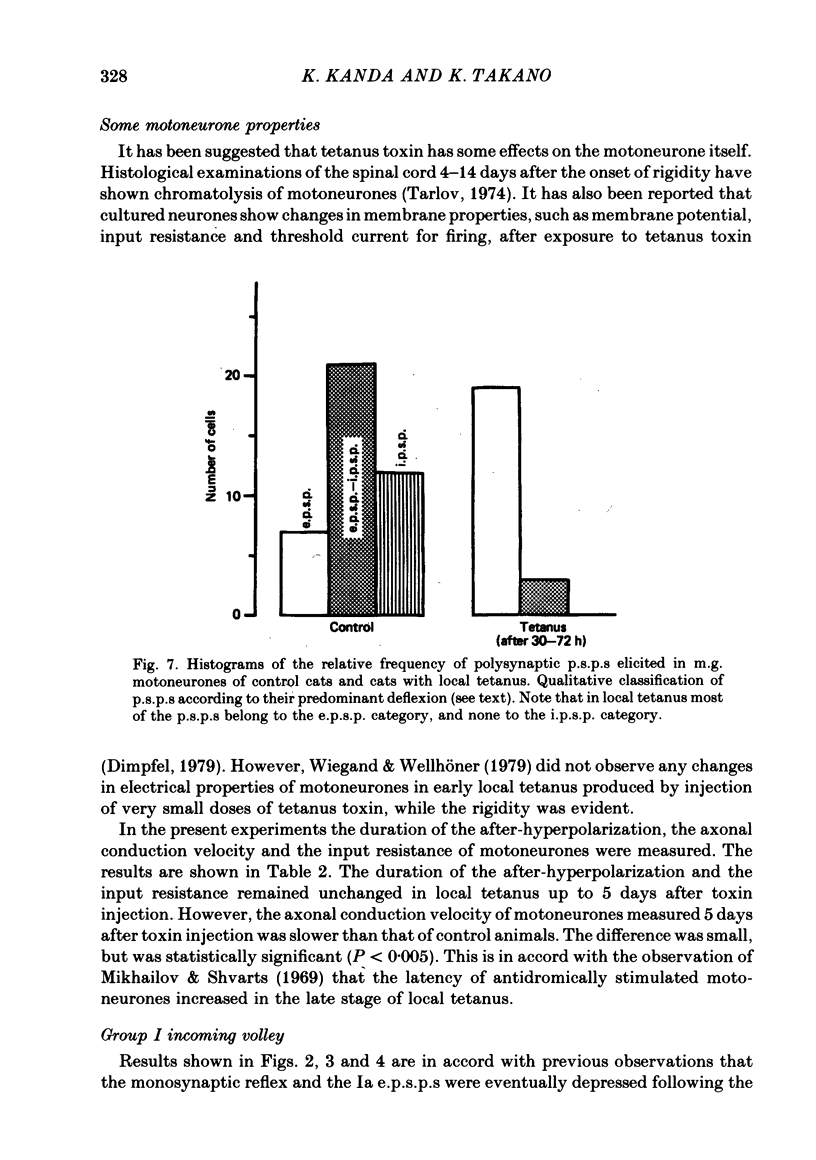
Tetanus toxin (100 mouse minimal lethal doses per kilogram) was injected into the medial gastrocnemius muscle of the cat. At various times thereafter, homonymous and heteronymous group Ia excitatory post-synaptic potentials (e.p.s.p.s), disynaptic reciprocal Ia inhibitory post-synaptic potentials (i.p.s.p.s) and post-synaptic potentials (p.s.p.s) produced by sural nerve stimulation were recorded in the medial gastrocnemius motoneurones. The duration of the after-hyperpolarization, the input resistance and the axonal conduction velocity of motoneurones were also measured. Homonymous Ia e.p.s.p.s remained normal until 72 h after toxin injection. However, 5 days after toxin injection, the amplitudes of Ia e.p.s.p.s. were significantly smaller than those in control animals (1.5 +/- 1.0 mV versus 5.6 +/- 2.7 mV; t test, P less than 0.001). Heteronymous Ia e.p.s.p.s produced by stimulation of the lateral gastrocnemius-soleus nerve 5 days after toxin injection were also significantly smaller than those in control animals (0.6 +/- 0.6 mV versus 2.5 +/- 1.5 mV; P less than 0.001). However, these heteronymous Ia e.p.s.p.s remained normal when the lateral gastrocnemius-soleus nerve was ligated and sectioned at the entry to those muscles just before the toxin injection. The ascending volleys, which are supposed to represent mainly the action potentials of the dorsal spinocerebellar tract and to be elicited monosynaptically by collaterals of group I afferents, were essentially the same in the left tetanic and right control sides up to 5 days after toxin injection. Ia i.p.s.p.s and the hyperpolarizing component of sural p.s.p.s could not be produced or were very small in motoneurones sampled later than 30 h after toxin injection. The duration of the after-hyperpolarization and the input resistance of motoneurones remained normal. Axonal conduction velocity of motoneurones measured 5 days after toxin injection was 89.4 +/- 12.7 m/s, and was significantly slower than that of control motoneurones (94.1 +/- 15.4 m/s) (P less than 0.005). Differences in the amplitude of group I incoming volleys between tetanic leg and contralateral control leg were not observed. These results suggest that tetanus toxin blocks excitatory synapses in the central nervous system as well as inhibitory synapses.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ambache N., Morgan R. S., Wright G. P. The action of tetanus toxin on the rabbit's iris. J Physiol. 1948 Jan 1;107(1):45–53. doi: 10.1113/jphysiol.1948.sp004248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BROOKS V. B., ASANUMA H. Action of tetanus toxin in the cerebral cortex. Science. 1962 Aug 31;137(3531):674–676. doi: 10.1126/science.137.3531.674-a. [DOI] [PubMed] [Google Scholar]
- BROOKS V. B., CURTIS D. R., ECCLES J. C. The action of tetanus toxin on the inhibition of motoneurones. J Physiol. 1957 Mar 11;135(3):655–672. doi: 10.1113/jphysiol.1957.sp005737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benecke R., Takano K., Schmidt J., Henatsch H. D. Tetanus toxin induced actions on spinal Renshaw cells and Ia-inhibitory interneurones during development of local tetanus in the cat. Exp Brain Res. 1977 Mar 30;27(3-4):271–286. doi: 10.1007/BF00235503. [DOI] [PubMed] [Google Scholar]
- Burke R. E., Jankowska E., ten Bruggencate G. A comparison of peripheral and rubrospinal synaptic input to slow and fast twitch motor units of triceps surae. J Physiol. 1970 May;207(3):709–732. doi: 10.1113/jphysiol.1970.sp009090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke R. E., Rymer W. Z. Relative strength of synaptic input from short-latency pathways to motor units of defined type in cat medial gastrocnemius. J Neurophysiol. 1976 May;39(3):447–458. doi: 10.1152/jn.1976.39.3.447. [DOI] [PubMed] [Google Scholar]
- Burke R. E., Strick P. L., Kanda K., Kim C. C., Walmsley B. Anatomy of medial gastrocnemius and soleus motor nuclei in cat spinal cord. J Neurophysiol. 1977 May;40(3):667–680. doi: 10.1152/jn.1977.40.3.667. [DOI] [PubMed] [Google Scholar]
- Curtis D. R., Felix D., Game C. J., McCulloch R. M. Tetanus toxin and the synaptic release of GABA. Brain Res. 1973 Mar 15;51:358–362. doi: 10.1016/0006-8993(73)90389-2. [DOI] [PubMed] [Google Scholar]
- Davies J., Tongroach P. Tetanus toxin and synaptic inhibition in the substantia nigra and striatum of the rat. J Physiol. 1979 May;290(2):23–36. doi: 10.1113/jphysiol.1979.sp012756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimpfel W. Hyperexcitability of cultured central nervous system neurons caused by tetanus toxin. Exp Neurol. 1979 Jul;65(1):53–65. doi: 10.1016/0014-4886(79)90247-4. [DOI] [PubMed] [Google Scholar]
- Duchen L. W. The effects of tetanus toxin on the motor end-plates of the mouse. An electron microscopic study. J Neurol Sci. 1973 Jun;19(2):153–167. doi: 10.1016/0022-510x(73)90159-7. [DOI] [PubMed] [Google Scholar]
- Duchen L. W., Tonge D. A. The effects of tetanus toxin on neuromuscular transmission and on the morphology of motor end-plates in slow and fast skeletal muscle of the mouse. J Physiol. 1973 Jan;228(1):157–172. doi: 10.1113/jphysiol.1973.sp010078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ECCLES J. C., KRNJEVIC K., MILEDI R. Delayed effects of peripheral severance of afferent nerve fibres on the efficacy of their central synapses. J Physiol. 1959 Jan 28;145(1):204–220. doi: 10.1113/jphysiol.1959.sp006136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erdmann G., Wiegand H., Wellhöner H. H. Intraaxonal and extraaxonal transport of 125I-tetanus toxin in early local tetanus. Naunyn Schmiedebergs Arch Pharmacol. 1975;290(4):357–373. doi: 10.1007/BF00499949. [DOI] [PubMed] [Google Scholar]
- Gushchin I. S., Kozhechkin S. N., Sverdlov Iu S. Giperpoliarizuiushchee deistvie glitsina na stolbniachnye motoneirony. Biull Eksp Biol Med. 1970 Aug;70(8):29–32. [PubMed] [Google Scholar]
- Kano M., Takano K. Gamma activity of the rigid cat caused by tetanus toxin. Jpn J Physiol. 1969 Feb 15;19(1):1–10. doi: 10.2170/jjphysiol.19.1. [DOI] [PubMed] [Google Scholar]
- Kretzschmar H., Kirchner F., Takano K. Relations between the effect of tetanus toxin on the neuromuscular transmission and histological functional properties of various muscles of the rat. Exp Brain Res. 1980 Jan;38(2):181–187. doi: 10.1007/BF00236739. [DOI] [PubMed] [Google Scholar]
- Kryzhanovskii G. N., Kurchavyi G. G., Sheikhon F. D. Supraspinal'nye vliianiia na motoneirony pri mestomon stolbniake. Biull Eksp Biol Med. 1973 Apr;75(4):36–39. [PubMed] [Google Scholar]
- Kryzhanovsky G. N. Present data on the pathogenesis of tetanus. Prog Drug Res. 1975;19:301–313. doi: 10.1007/978-3-0348-7090-0_34. [DOI] [PubMed] [Google Scholar]
- Kryzhanovsky G. N. The mechanism of action of tetanus toxin: effect on synaptic processes and some particular features of toxin binding by the nervous tissue. Naunyn Schmiedebergs Arch Pharmacol. 1973;276(3-4):247–270. doi: 10.1007/BF00499880. [DOI] [PubMed] [Google Scholar]
- LAPORTE Y., LUNDBERG A., OSCARSSON O. Functional organization of the dorsal spino-cerebellar tract in the cat. I. Recording of mass discharge in dissected Flechsig's fasciculus. Acta Physiol Scand. 1956 Mar 24;36(1-2):175–187. doi: 10.1111/j.1748-1716.1956.tb01316.x. [DOI] [PubMed] [Google Scholar]
- LAPORTE Y., LUNDBERG A., OSCARSSON O. Functional organization of the dorsal spino-cerebellar tract in the cat. II. Single fibre recording in Flechsig's fasciculus on electrical stimulation of various peripheral nerves. Acta Physiol Scand. 1956 Mar 24;36(1-2):188–203. doi: 10.1111/j.1748-1716.1956.tb01317.x. [DOI] [PubMed] [Google Scholar]
- LLOYD D. P. C., McINTYRE A. K. Dorsal column conduction of group I muscle afferent impulses and their relay through Clarke's column. J Neurophysiol. 1950 Jan;13(1):39–54. doi: 10.1152/jn.1950.13.1.39. [DOI] [PubMed] [Google Scholar]
- Mellanby J., Green J. How does tetanus toxin act? Neuroscience. 1981;6(3):281–300. doi: 10.1016/0306-4522(81)90123-8. [DOI] [PubMed] [Google Scholar]
- Mikhailov V. V., Shvarts I. L. Mikrofiziologicheskii analiz élektricheskoi aktivnosti razlichnykh tipov spinal'nykh neironov pri éksperimental'nom stolbniake. Biull Eksp Biol Med. 1969 Dec;68(12):20–23. [PubMed] [Google Scholar]
- Nelson P. G., Frank K. Anomalous rectification in cat spinal motoneurons and effect of polarizing currents on excitatory postsynaptic potential. J Neurophysiol. 1967 Sep;30(5):1097–1113. doi: 10.1152/jn.1967.30.5.1097. [DOI] [PubMed] [Google Scholar]
- Price D. L., Griffin J. W., Peck K. Tetanus toxin: evidence for binding at presynaptic nerve endings. Brain Res. 1977 Feb;121(2):379–384. doi: 10.1016/0006-8993(77)90163-9. [DOI] [PubMed] [Google Scholar]
- Price D. L., Griffin J., Young A., Peck K., Stocks A. Tetanus toxin: direct evidence for retrograde intraaxonal transport. Science. 1975 May 30;188(4191):945–947. doi: 10.1126/science.49080. [DOI] [PubMed] [Google Scholar]
- Schwab M. E., Thoenen H. Electron microscopic evidence for a transsynaptic migration of tetanus toxin in spinal cord motoneurons: an autoradiographic and morphometric study. Brain Res. 1976 Mar 26;105(2):213–227. doi: 10.1016/0006-8993(76)90422-4. [DOI] [PubMed] [Google Scholar]
- Stöckel K., Schwab M., Thoenen H. Comparison between the retrograde axonal transport of nerve growth factor and tetanus toxin in motor, sensory and adrenergic neurons. Brain Res. 1975 Nov 28;99(1):1–16. doi: 10.1016/0006-8993(75)90604-6. [DOI] [PubMed] [Google Scholar]
- Tarlov I. M. Rigidity and primary motoneuron damage in tetanus. Exp Neurol. 1974 Aug;44(2):246–254. doi: 10.1016/0014-4886(74)90062-4. [DOI] [PubMed] [Google Scholar]
- Wiegand H., Wellhöner H. H. Electrical excitability of motoneurones in early local tetanus. Naunyn Schmiedebergs Arch Pharmacol. 1979 Jul;308(1):71–76. doi: 10.1007/BF00499722. [DOI] [PubMed] [Google Scholar]


