Abstract
1. Segmental, lumbar sympathetic outflow to neurones in the cat inferior mesenteric ganglion and to the large intestine were studied. Synaptic responses of neurones in the inferior mesenteric ganglion were recorded intracellularly, in vitro, during electrical stimulation of preganglionic fibres in the lumbar white rami. Synaptic responses consisted of excitatory post-synaptic potentials and/or action potentials.
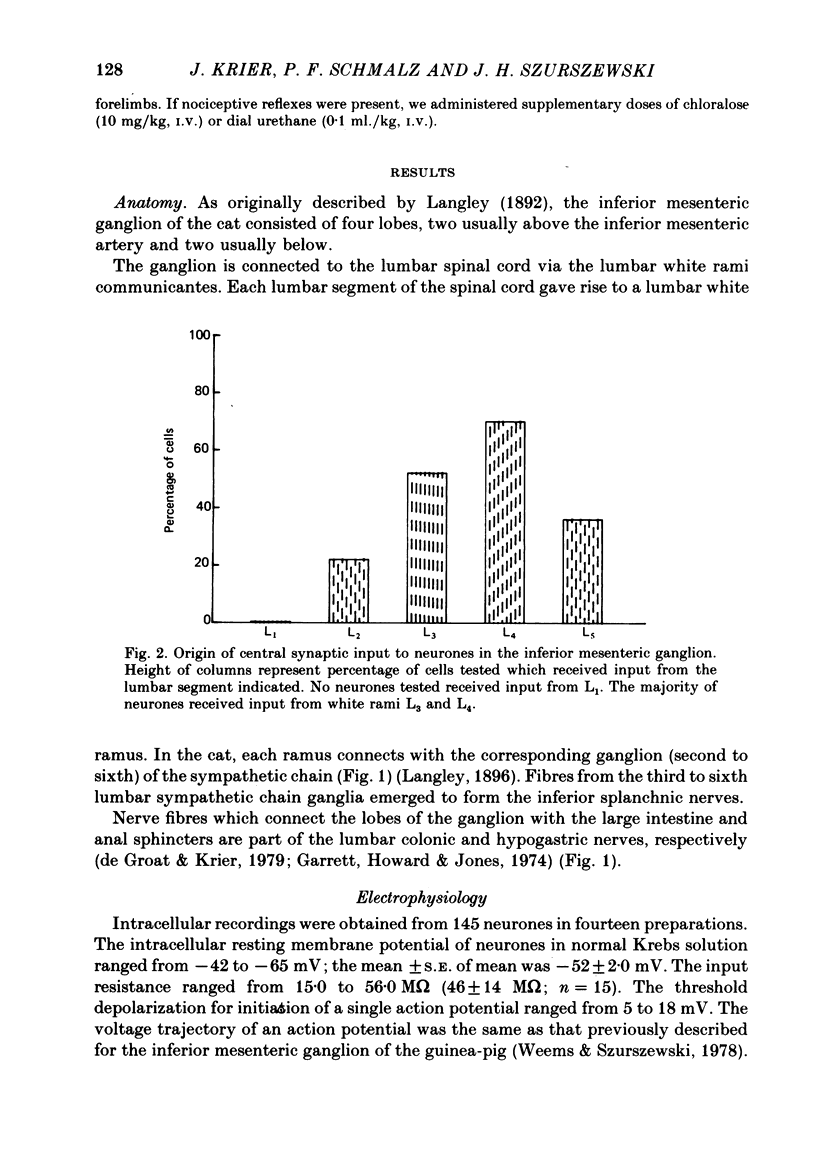
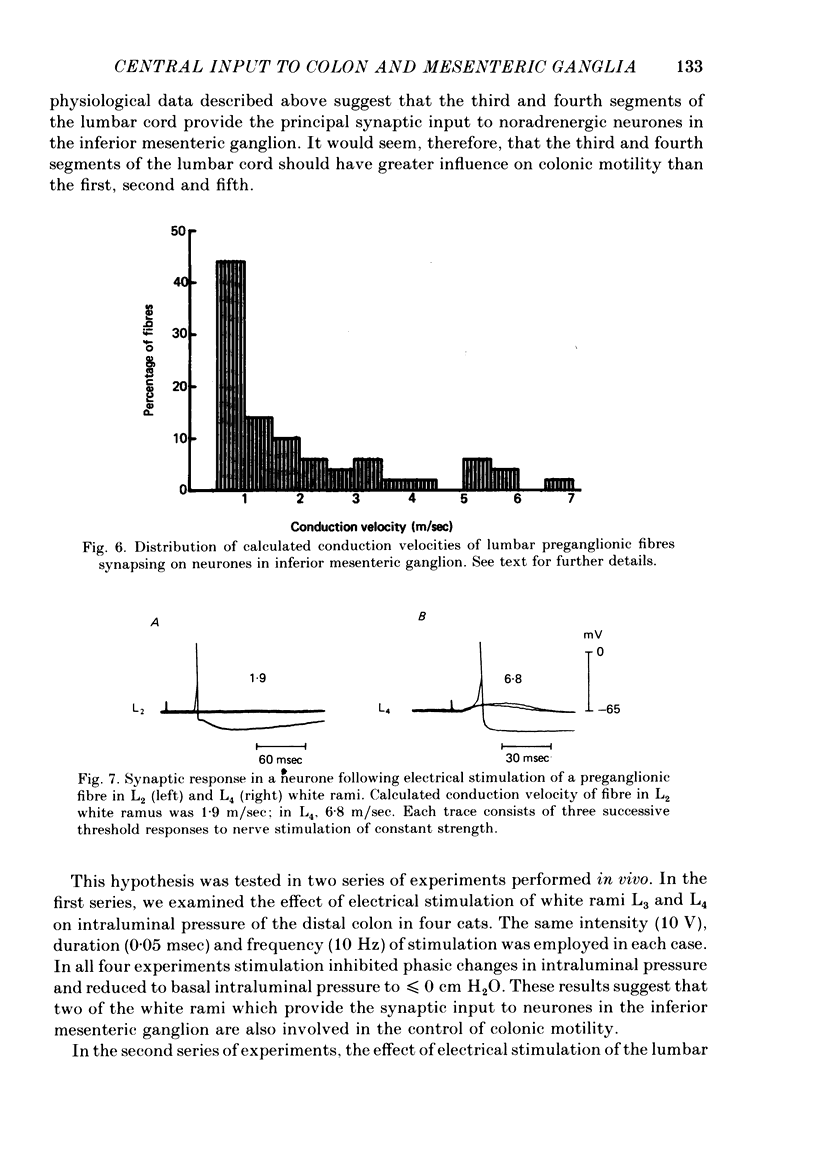
2. None of the neurones tested received synaptic input from spinal cord segment L1. There was synaptic input from segments L2-L5 of the spinal cord. The strongest synaptic input arose from spinal cord segments L3 and L4.
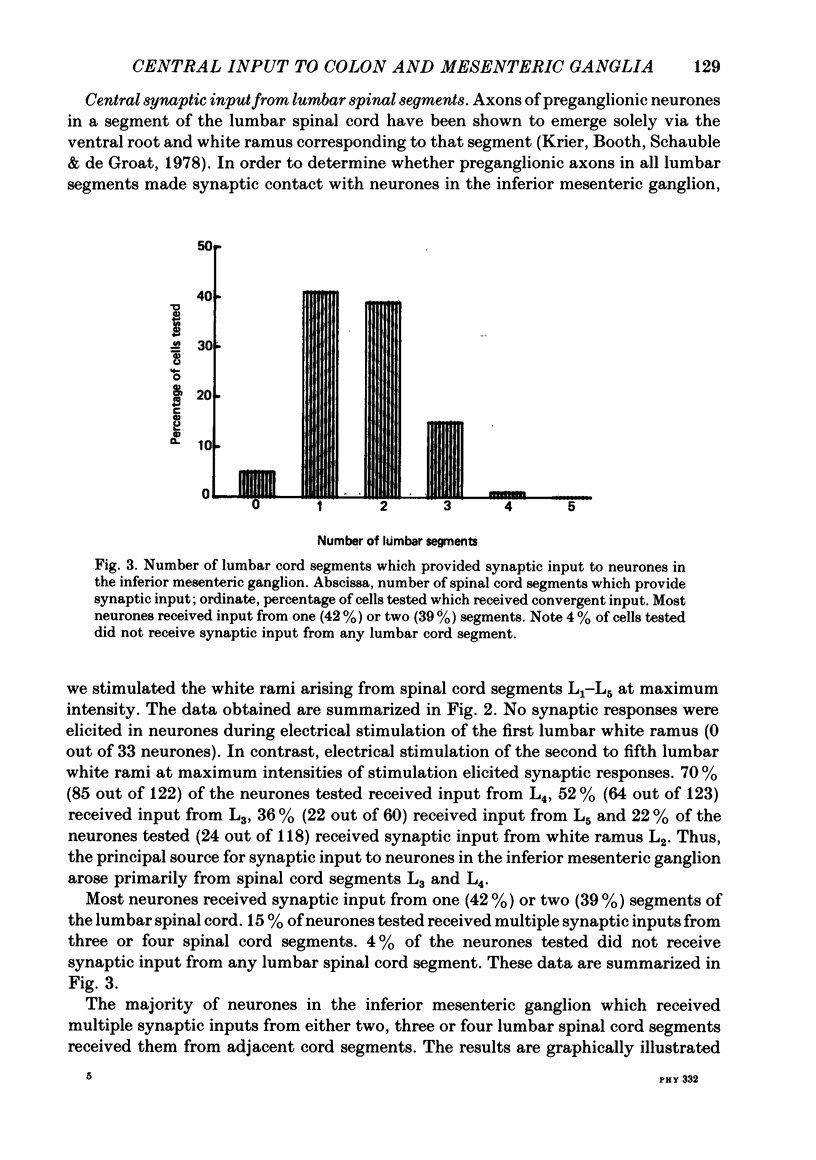
3. 42% of the neurones tested received synaptic input from only one spinal cord segment. 54% of the neurones tested received convergent synaptic input from two, three or four adjacent lumbar segments.
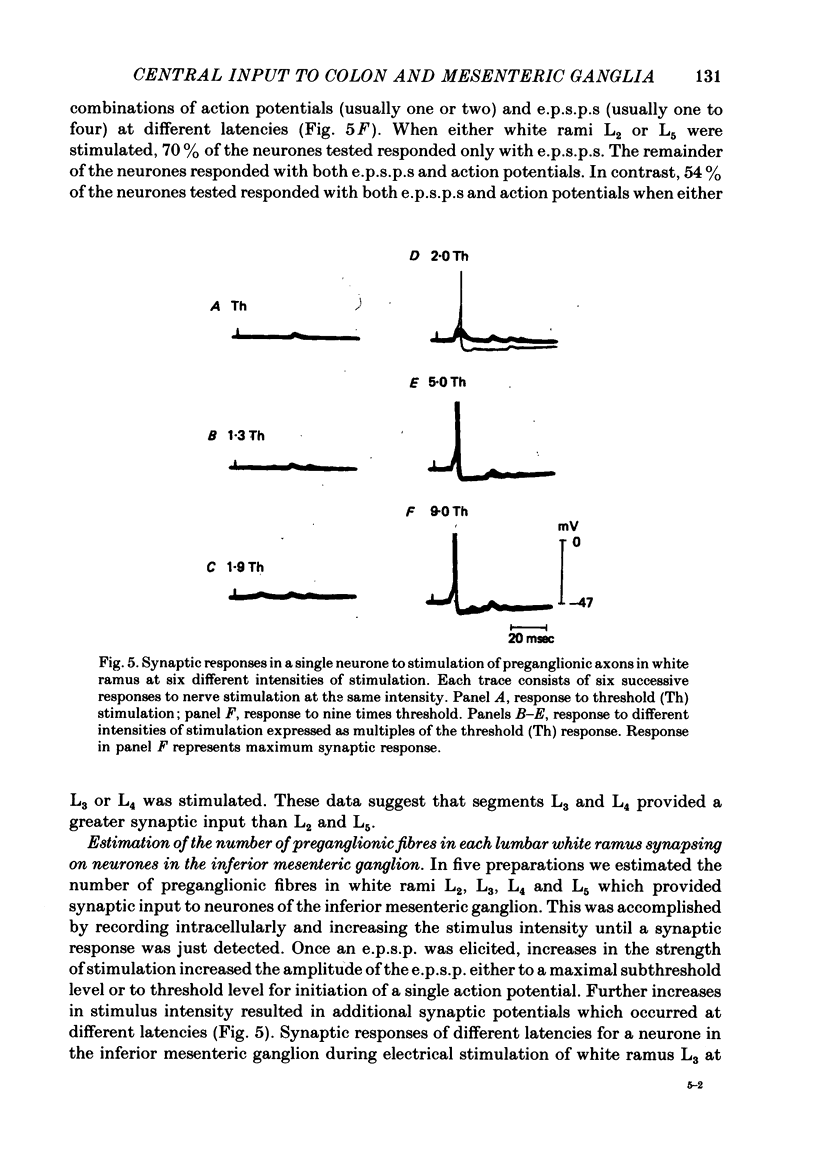
4. Electrophysiological measurements indicated that the number of preganglionic fibres in any lumbar white ramus communicans which provided synaptic input ranged from one to thirteen. Each lumbar white ramus contained, on average, five preganglionic fibres which provided synaptic input to neurones in the inferior mesenteric ganglion.
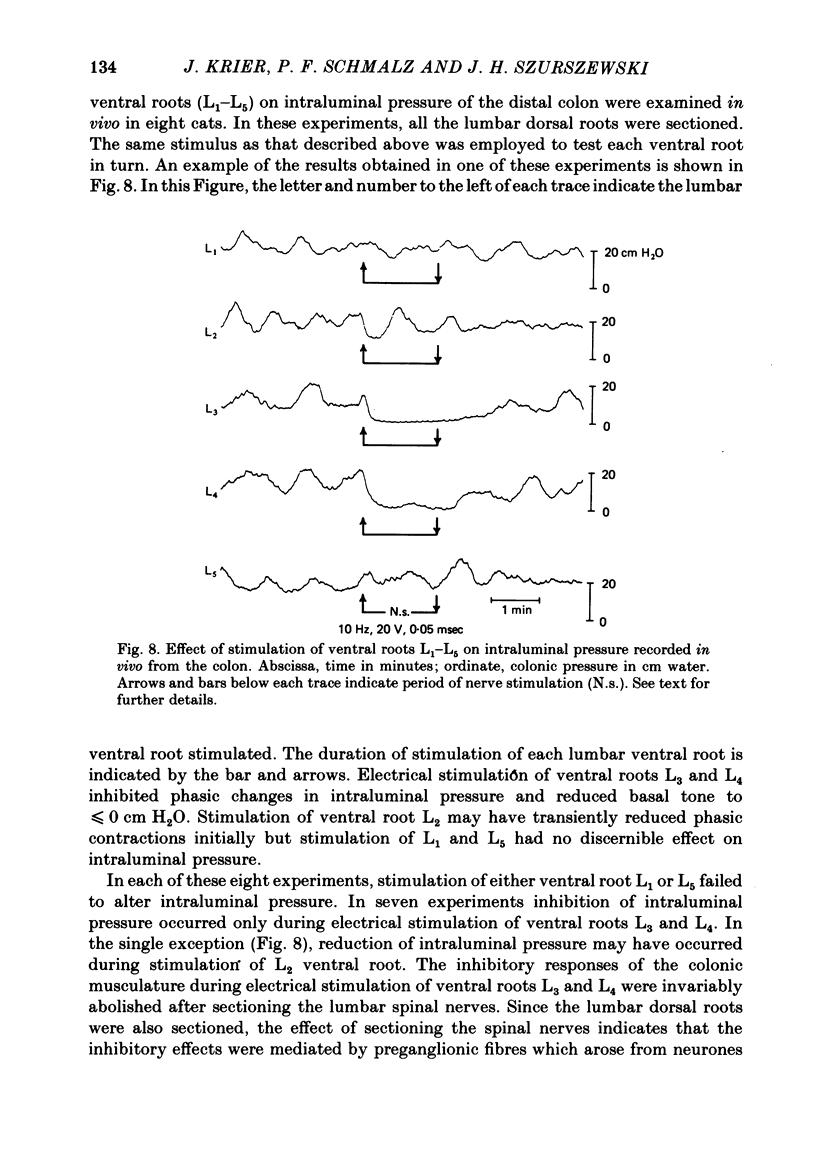
5. Changes in intraluminal colonic pressure were measured in vivo during electrical stimulation of preganglionic fibres in the different lumbar white rami and lumbar ventral roots. Electrical stimulation of white rami L3 and L4 abolished phasic changes in intraluminal colonic pressure and reduced basal pressure to near zero. Electrical stimulation of preganglionic fibres in lumbar ventral roots L3 and L4 abolished phasic changes in intraluminal colonic pressure and reduced basal pressure to near zero. Stimulation of ventral roots L1, L2 and L5 had little to no effect on intraluminal pressure.
6. Based on the data obtained in this study, two hypotheses are proposed. First, spinal cord segments L3, L4 and L5 are the primary sources of central synaptic input to neurones in the inferior mesenteric ganglion. Secondly, spinal cord segments L3 and L4 control colonic motility.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brooksby G. A., Donald D. E. Sympathetic outflow from spinal cord to splanchnic circulation of the dog. Am J Physiol. 1970 Nov;219(5):1429–1433. doi: 10.1152/ajplegacy.1970.219.5.1429. [DOI] [PubMed] [Google Scholar]
- Crowcroft P. J., Szurszewski J. H. A study of the inferior mesenteric and pelvic ganglia of guinea-pigs with intracellular electrodes. J Physiol. 1971 Dec;219(2):421–441. doi: 10.1113/jphysiol.1971.sp009670. [DOI] [PMC free article] [PubMed] [Google Scholar]
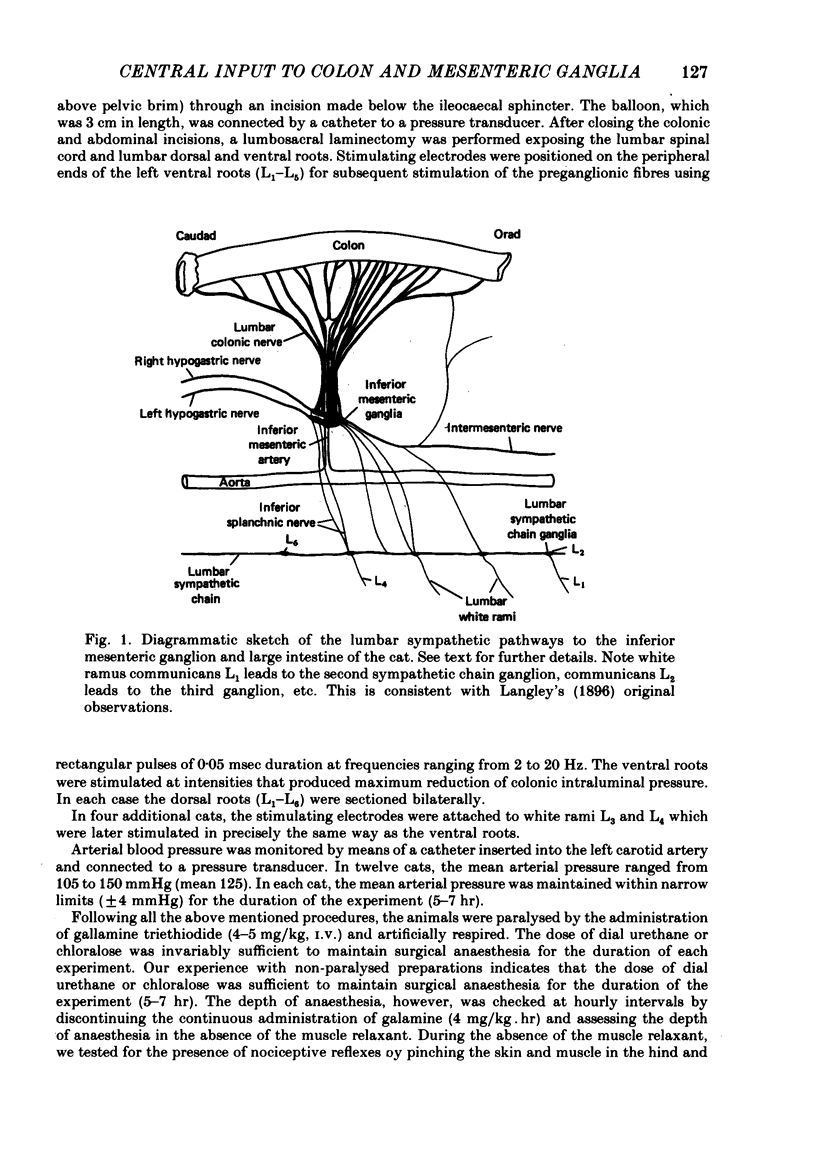
- De Groat W. C., Krier J. The central control of the lumbar sympathetic pathway to the large intestine of the cat. J Physiol. 1979 Apr;289:449–468. doi: 10.1113/jphysiol.1979.sp012746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erulkar S. D., Woodward J. K. Intracellular recording from mammalian superior cervical ganglion in situ. J Physiol. 1968 Nov;199(1):189–203. doi: 10.1113/jphysiol.1968.sp008648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrett J. R., Howard E. R., Jones W. The internal anal sphincter in the cat: a study of nervous mechanisms affecting tone and reflex activity. J Physiol. 1974 Nov;243(1):153–166. doi: 10.1113/jphysiol.1974.sp010747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garry R. C. The nervous control of the caudal region of the large bowel in the cat. J Physiol. 1933 Mar 15;77(4):422–431. doi: 10.1113/jphysiol.1933.sp002977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreulen D. L., Szurszewski J. H. Nerve pathways in celiac plexus of the guinea pig. Am J Physiol. 1979 Jul;237(1):E90–E97. doi: 10.1152/ajpendo.1979.237.1.E90. [DOI] [PubMed] [Google Scholar]
- Langley J. N., Anderson H. K. On the Innervation of the Pelvic and Adjoining Viscera: Part I. The Lower Portion of the Intestine. J Physiol. 1895 May 20;18(1-2):67–105. doi: 10.1113/jphysiol.1895.sp000558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langley J. N., Anderson H. K. The Innervation of the Pelvic and adjoining Viscera: Part VII. Anatomical Observations. J Physiol. 1896 Oct 19;20(4-5):372–406. doi: 10.1113/jphysiol.1896.sp000629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langley J. N. Note on Regeneration of Prae-Ganglionic Fibres of the Sympathetic. J Physiol. 1895 Jul 18;18(3):280–284. doi: 10.1113/jphysiol.1895.sp000566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langley J. N. Observations on the Medullated Fibres of the Sympathetic System and chiefly on those of the Grey Rami Communicantes. J Physiol. 1896 Jun 6;20(1):55–76. doi: 10.1113/jphysiol.1896.sp000611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtman J. W., Purves D., Yip J. W. Innervation of sympathetic neurones in the guinea-pig thoracic chain. J Physiol. 1980 Jan;298:285–299. doi: 10.1113/jphysiol.1980.sp013081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirgorodsky V. N., Skok V. I. Intracellular potentials recorded from a tonically active mammalian sympathetic ganglion. Brain Res. 1969 Oct;15(2):570–572. doi: 10.1016/0006-8993(69)90187-5. [DOI] [PubMed] [Google Scholar]
- Njå A., Purves D. Specific innervation of guinea-pig superior cervical ganglion cells by preganglionic fibres arising from different levels of the spinal cord. J Physiol. 1977 Jan;264(2):565–583. doi: 10.1113/jphysiol.1977.sp011683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perri V., Sacchi O., Caella C. Electrical properties and synaptic connections of the sympathetic neurons in the rat and guinea-pig superior cervical ganglion. Pflugers Arch. 1970;314(1):40–54. doi: 10.1007/BF00587045. [DOI] [PubMed] [Google Scholar]
- Sonnenschein R. R., Weissman M. L. Sympathetic vasomotor outflows to hindlimb muscles of the cat. Am J Physiol. 1978 Nov;235(5):H482–H487. doi: 10.1152/ajpheart.1978.235.5.H482. [DOI] [PubMed] [Google Scholar]
- Wallis D. I., North R. A. Synaptic input to cells of the rabbit superior cervical ganglion. Pflugers Arch. 1978 May 18;374(2):145–152. doi: 10.1007/BF00581295. [DOI] [PubMed] [Google Scholar]
- Weems W. A., Szurszewski J. H. An intracellular analysis of some intrinsic factors controlling neural output from inferior mesenteric ganglion of guinea pigs. J Neurophysiol. 1978 Mar;41(2):305–321. doi: 10.1152/jn.1978.41.2.305. [DOI] [PubMed] [Google Scholar]
- Weems W. A., Szurszewski J. H. Modulation of colonic motility by peripheral neural inputs to neurons of the inferior mesenteric ganglion. Gastroenterology. 1977 Aug;73(2):273–278. [PubMed] [Google Scholar]
- Weight F. F., Votava J. Slow synaptic excitation in sympathetic ganglion cells: evidence for synaptic inactivation of potassium conductance. Science. 1970 Nov 13;170(3959):755–758. doi: 10.1126/science.170.3959.755. [DOI] [PubMed] [Google Scholar]
- de Groat W. C., Krier J. An electrophysiological study of the sacral parasympathetic pathway to the colon of the cat. J Physiol. 1976 Sep;260(2):425–445. doi: 10.1113/jphysiol.1976.sp011523. [DOI] [PMC free article] [PubMed] [Google Scholar]


