Abstract
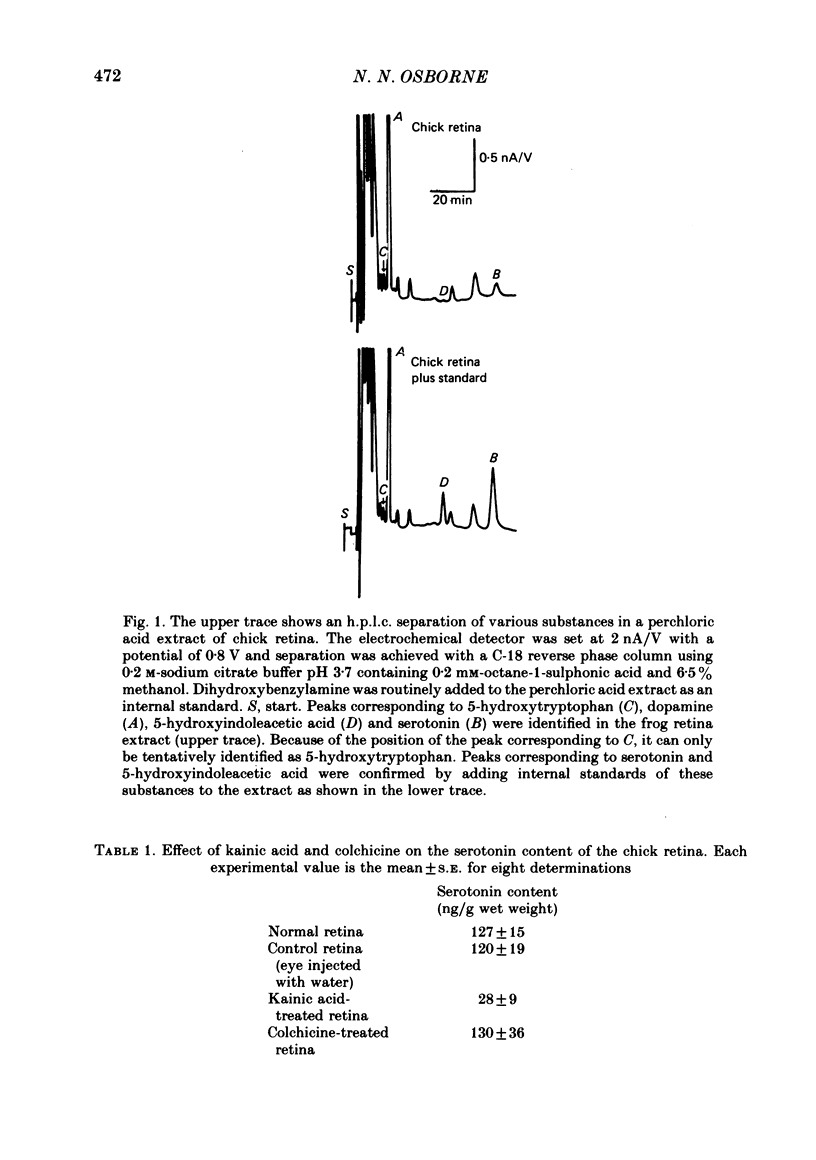
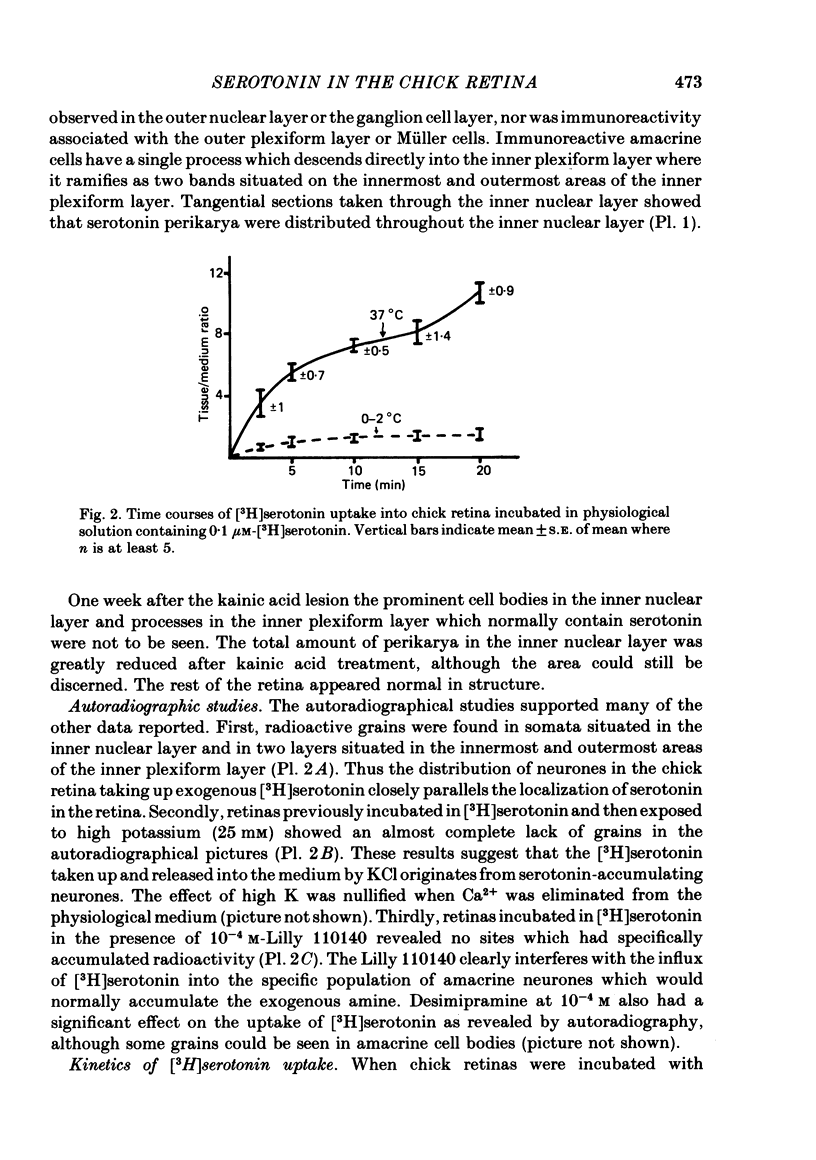
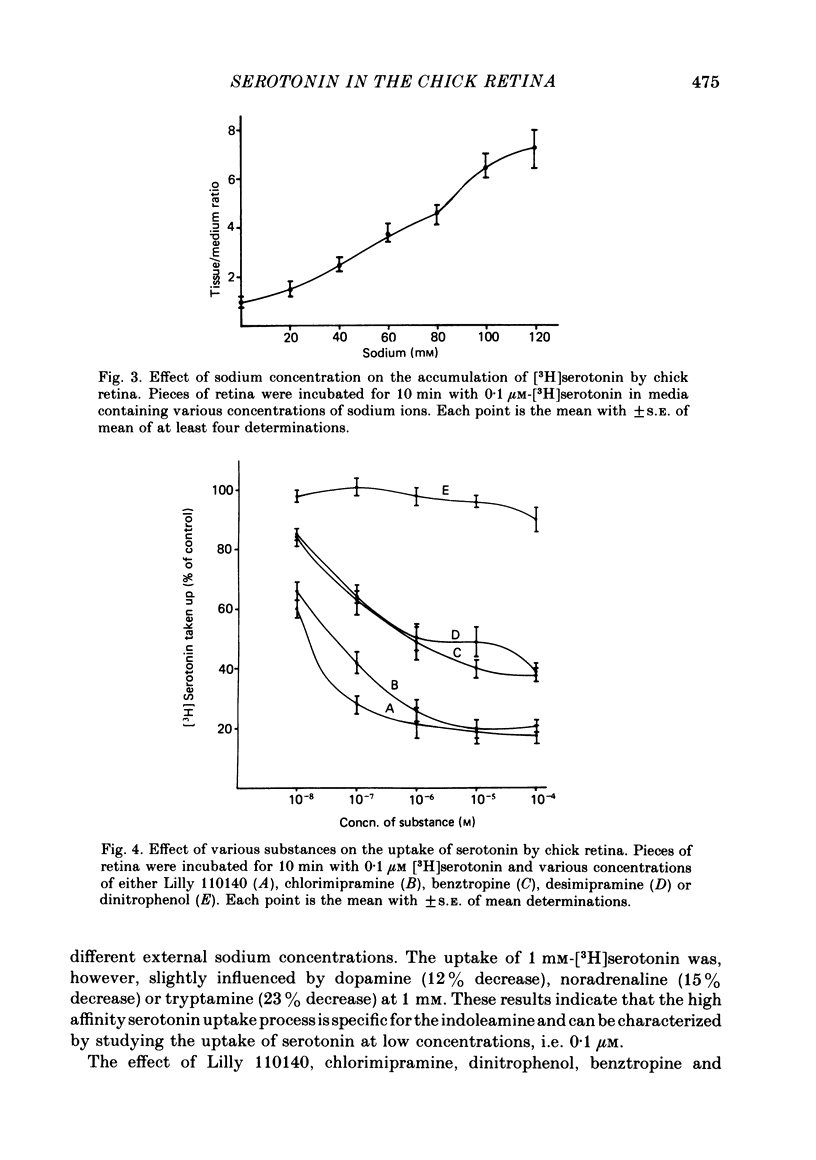
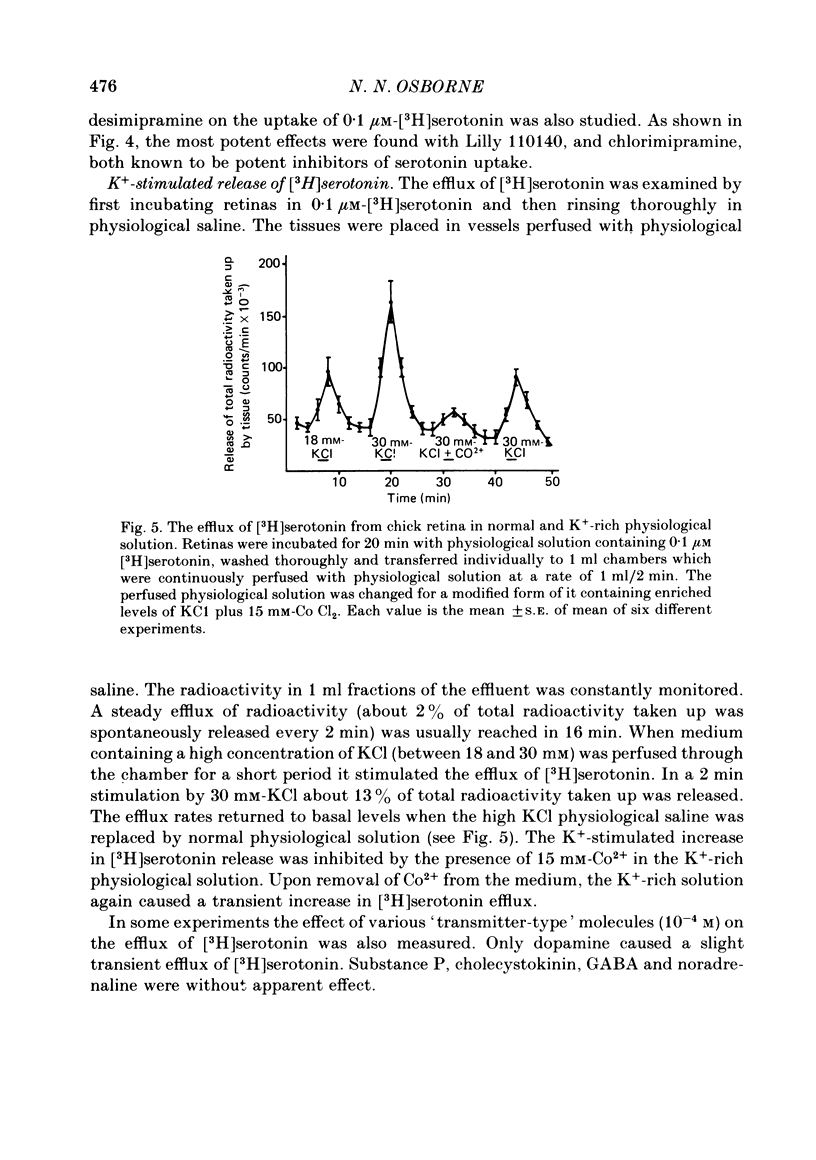
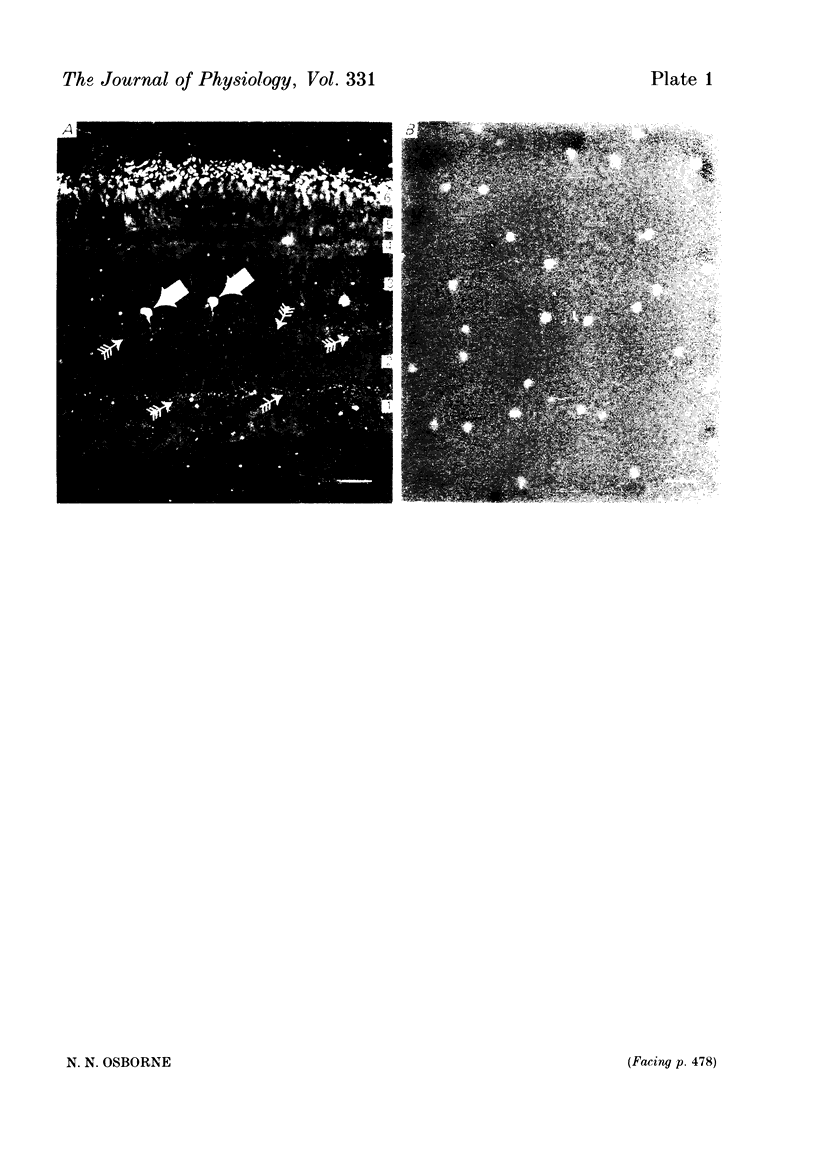
1. High pressure liquid chromatography and electrochemical detection have shown that serotonin exists in the chick retina (127 ng/g wet weight). No other indoleamine was identified. 2. Immunofluorescent histological studies showed that the endogenous serotonin was localized apparently in those cell bodies and processes which took up exogenous [3H]serotonin as revealed by autoradiography. These serotonergic neurones can be destroyed by injecting kainic acid into the eye. 3. Isolated chick retina accumulated exogenous [3H]serotonin. Kinetic analysis revealed the presence of two saturable uptake systems: a 'high affinity' mechanism with an apparent Km of 5.9 X 10(-8) M and a Vmax of 0.143 X 10(-13) mol/mg wet weight . min and a low affinity mechanism with an apparent Km2 of 1.8 X 10(-3) M and Vmax of 0.12 X 10(-9) mol/mg wet weight . min. 4. The uptake of serotonin was temperature-sensitive and sodium-dependent and Lilly 110140 and chlorimipramine were potent inhibitors of the amine uptake. 5. Autoradiographic studies indicated that neuronal processes associated with the innermost and outermost areas of the inner plexiform layer and perikarya situated in the inner nuclear layer are the sites which accumulated exogenous [3H]serotonin. 6. [3H]Serotonin accumulated in the retina was released by increasing the external K+ concentration. This release was Ca2+-dependent. Additionally, autoradiographic studies show that [3H]serotonin taken up by the serotonin neurones was also released by Ca2+-dependent K+ depolarization of the retina.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alam M., Smirk F. H. Observations in man on a pulse-accelerating reflex from the voluntary muscles of the legs. J Physiol. 1938 Mar 14;92(2):167–177. doi: 10.1113/jphysiol.1938.sp003592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appelberg B., Johansson H., Kalistratov G. The influence of group II muscle afferents and low threshold skin afferents on dynamic fusimotor neurones to the triceps surae of the cat. Brain Res. 1977 Aug 19;132(1):153–158. doi: 10.1016/0006-8993(77)90713-2. [DOI] [PubMed] [Google Scholar]
- Appenteng K., Morimoto T., Taylor A. Fusimotor activity in masseter nerve of the cat during reflex jaw movements. J Physiol. 1980 Aug;305:415–431. doi: 10.1113/jphysiol.1980.sp013373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker P. C., Hoff K. M. Daily variation in the eye's 5-HT stores. Experientia. 1979 Jun 15;35(6):780–780. doi: 10.1007/BF01968243. [DOI] [PubMed] [Google Scholar]
- Bigland-Ritchie B. EMG and fatigue of human voluntary and stimulated contractions. Ciba Found Symp. 1981;82:130–156. doi: 10.1002/9780470715420.ch9. [DOI] [PubMed] [Google Scholar]
- Buckerfield M., Oliver J., Chubb I. W., Morgan I. G. Somatostatin-like immunoreactivity in amacrine cells of the chicken retina. Neuroscience. 1981;6(4):689–695. doi: 10.1016/0306-4522(81)90152-4. [DOI] [PubMed] [Google Scholar]
- COOPER S. The responses of the primary and secondary endings of muscle spindles with intact motor innervation during applied stretch. Q J Exp Physiol Cogn Med Sci. 1961 Oct;46:389–398. doi: 10.1113/expphysiol.1961.sp001558. [DOI] [PubMed] [Google Scholar]
- Consolazione A., Milstein C., Wright B., Cuello A. C. Immunocytochemical detection of serotonin with monoclonal antibodies. J Histochem Cytochem. 1981 Dec;29(12):1425–1430. doi: 10.1177/29.12.7033368. [DOI] [PubMed] [Google Scholar]
- Coote J. H., Hilton S. M., Perez-Gonzalez J. F. The reflex nature of the pressor response to muscular exercise. J Physiol. 1971 Jul;215(3):789–804. doi: 10.1113/jphysiol.1971.sp009498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehinger B., Florén I. Quantitation of the uptake of indoleamines and dopamine in the rabbit retina. Exp Eye Res. 1978 Jan;26(1):1–11. doi: 10.1016/0014-4835(78)90147-1. [DOI] [PubMed] [Google Scholar]
- Ellaway P. H. An application of cumulative sum technique (cusums) to neurophysiology [proceedings]. J Physiol. 1977 Feb;265(1):1P–2P. [PMC free article] [PubMed] [Google Scholar]
- Ellaway P. H. Cumulative sum technique and its application to the analysis of peristimulus time histograms. Electroencephalogr Clin Neurophysiol. 1978 Aug;45(2):302–304. doi: 10.1016/0013-4694(78)90017-2. [DOI] [PubMed] [Google Scholar]
- Ellaway P. H., Murphy P. R. A comparison of the recurrent inhibition of alpha- and gamma-motoneurones in the cat. J Physiol. 1981 Jun;315:43–58. doi: 10.1113/jphysiol.1981.sp013731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellaway P. H., Murphy P. R. Autogenetic effects of muscle contraction on extensor gamma motoneurones in the cat. Exp Brain Res. 1980 Feb;38(3):305–312. doi: 10.1007/BF00236650. [DOI] [PubMed] [Google Scholar]
- Ellaway P. H., Murphy P. R., Trott J. R. Inhibition of gamma motoneurone discharge by contraction of the homonymous muscle in the decerebrated cat. J Physiol. 1979 Jun;291:425–441. doi: 10.1113/jphysiol.1979.sp012823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellaway P. H. Recurrent inhibition of fusimotor neurones exhibiting background discharges in the decerebrate and the spinal cat. J Physiol. 1971 Jul;216(2):419–439. doi: 10.1113/jphysiol.1971.sp009533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellaway P. H., Trott J. R. Autogenetic reflex action on to gamma motoneurones by stretch of triceps surae in the decerebrated cat. J Physiol. 1978 Mar;276:49–66. doi: 10.1113/jphysiol.1978.sp012219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Florén I., Hansson H. C. Investigations into whether 5-hydroxytryptamine is a neurotransmitter in the retina of rabbit and chicken. Invest Ophthalmol Vis Sci. 1980 Feb;19(2):117–125. [PubMed] [Google Scholar]
- Floyd K., McMahon S. B., Morrison J. F. Inhibitory interactions between colonic and vesical afferents in the micturition reflex of the cat. J Physiol. 1982 Jan;322:45–52. doi: 10.1113/jphysiol.1982.sp014021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu T. C., Schomburg E. D. Electrophysiological investigation of the projection of secondary muscle spindle afferents in the cat spinal cord. Acta Physiol Scand. 1974 Jul;91(3):314–329. doi: 10.1111/j.1748-1716.1974.tb05687.x. [DOI] [PubMed] [Google Scholar]
- Ghez C., Shinoda Y. Spinal mechanisms of the functional stretch reflex. Exp Brain Res. 1978 May 12;32(1):55–68. doi: 10.1007/BF00237390. [DOI] [PubMed] [Google Scholar]
- Grillner S., Hongo T., Lund S. Descending monosynaptic and reflex control of gamma-motoneurones. Acta Physiol Scand. 1969 Apr;75(4):592–613. doi: 10.1111/j.1748-1716.1969.tb04414.x. [DOI] [PubMed] [Google Scholar]
- HUNT C. C., PAINTAL A. S. Spinal reflex regulation of fusimotor neurones. J Physiol. 1958 Sep 23;143(2):195–212. doi: 10.1113/jphysiol.1958.sp006053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUNT C. C. Relation of function to diameter in afferent fibers of muscle nerves. J Gen Physiol. 1954 Sep 20;38(1):117–131. doi: 10.1085/jgp.38.1.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauschild D. C., Laties A. M. An indoleamine-containing cell in chick retina. Invest Ophthalmol. 1973 Jul;12(7):537–540. [PubMed] [Google Scholar]
- Kniffki K. D., Mense S., Schmidt R. F. Responses of group IV afferent units from skeletal muscle to stretch, contraction and chemical stimulation. Exp Brain Res. 1978 Apr 14;31(4):511–522. doi: 10.1007/BF00239809. [DOI] [PubMed] [Google Scholar]
- Kniffki K. D., Mense S., Schmidt R. F. The spinocervical tract as a possible pathway for muscular nociception. J Physiol (Paris) 1977 Sep;73(3):359–366. [PubMed] [Google Scholar]
- Mense S. Nervous outflow from skeletal muscle following chemical noxious stimulation. J Physiol. 1977 May;267(1):75–88. doi: 10.1113/jphysiol.1977.sp011802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neal M. J. Amino acid transmitter substances in the vertebrate retina. Gen Pharmacol. 1976 Oct;7(5):321–332. doi: 10.1016/0306-3623(76)90014-8. [DOI] [PubMed] [Google Scholar]
- Noth J., Thilmann A. Autogenetic excitation of extensor gamma-motoneurones by group II muscle afferents in the cat. Neurosci Lett. 1980 Apr;17(1-2):23–26. doi: 10.1016/0304-3940(80)90055-5. [DOI] [PubMed] [Google Scholar]
- Osborne N. N. In vitro experiments on the metabolism, uptake and release of 5-hydroxytryptamine in bovine retina. Brain Res. 1980 Feb 24;184(2):283–297. doi: 10.1016/0006-8993(80)90799-4. [DOI] [PubMed] [Google Scholar]
- Osborne N. N., Nicholas D. A., Dockray G. J., Cuello A. C. Cholecystokinin and substance P immunoreactivity in retinas of rats, frogs, lizards and chicks. Exp Eye Res. 1982 Apr;34(4):639–649. doi: 10.1016/0014-4835(82)90038-0. [DOI] [PubMed] [Google Scholar]
- Osborne N. N. Noradrenaline, a transmitter candidate in the retina. J Neurochem. 1981 Jan;36(1):17–27. doi: 10.1111/j.1471-4159.1981.tb02372.x. [DOI] [PubMed] [Google Scholar]
- Osborne N. N., Richardson G. Specificity of serotonin uptake by bovine retina: comparison with tryptamine. Exp Eye Res. 1980 Jul;31(1):31–39. doi: 10.1016/0014-4835(80)90088-3. [DOI] [PubMed] [Google Scholar]
- PAINTAL A. S. Functional analysis of group III afferent fibres of mammalian muscles. J Physiol. 1960 Jul;152:250–270. doi: 10.1113/jphysiol.1960.sp006486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rymer W. Z., Houk J. C., Crago P. E. Mechanisms of the clasp-knife reflex studied in an animal model. Exp Brain Res. 1979 Sep;37(1):93–113. doi: 10.1007/BF01474257. [DOI] [PubMed] [Google Scholar]
- Sarthy P. V., Lam D. M. The uptake and release of [3H]dopamine in the goldfish retina. J Neurochem. 1979 Apr;32(4):1269–1277. doi: 10.1111/j.1471-4159.1979.tb11054.x. [DOI] [PubMed] [Google Scholar]
- Shaskan E. G., Snyder S. H. Kinetics of serotonin accumulation into slices from rat brain: relationship to catecholamine uptake. J Pharmacol Exp Ther. 1970 Nov;175(2):404–418. [PubMed] [Google Scholar]
- Suzuki O., Noguchi E., Yagi K. Uptake of 5-hydroxytryptamine by chick retina. J Neurochem. 1978 Jan;30(1):295–296. doi: 10.1111/j.1471-4159.1978.tb07069.x. [DOI] [PubMed] [Google Scholar]
- Tanji J., Kato M. The long-lasting effects of cutaneous and high threshold muscle afferent volleys on semitendinosus -motoneurones. Brain Res. 1972 May 26;40(2):523–526. doi: 10.1016/0006-8993(72)90156-4. [DOI] [PubMed] [Google Scholar]
- Weakly J. N. The action of cobalt ions on neuromuscular transmission in the frog. J Physiol. 1973 Nov;234(3):597–612. doi: 10.1113/jphysiol.1973.sp010363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong D. T., Horng J. S., Bymaster F. P., Hauser K. L., Molloy B. B. A selective inhibitor of serotonin uptake: Lilly 110140, 3-(p-trifluoromethylphenoxy)-N-methyl-3-phenylpropylamine. Life Sci. 1974 Aug 1;15(3):471–479. doi: 10.1016/0024-3205(74)90345-2. [DOI] [PubMed] [Google Scholar]




