Abstract
1. Methionyl-t-RNA synthetase (where t-RNA denotes `soluble' or transfer RNA) has been purified to apparent homogeneity from a ribonuclease I-free strain of Escherichia coli. Polyacrylamide-gel electrophoresis of the final product revealed a single band. The purified enzyme catalyses the exchange of 450μmoles of pyrophosphate into ATP/mg. in 15min. at 37°. 2. Methionyl-t-RNA synthetase is specific for the l-isomer of methionine, but appears to catalyse the methionylation of two distinct species of t-RNA, both of which are specific for methionine, but only one of which may be subsequently formylated. 3. The Michaelis constant for l-methionine is 2×10−4m in the ATP–PPi exchange assay and 2×10−5m for the acylation of t-RNA. 4. Gel filtration of both crude and highly purified preparations of methionyl-t-RNA synthetase on Sephadex G-200 indicates that the active species of enzyme has a molecular weight of about 190000. The amino acid composition of the enzyme is similar to those reported for the isoleucine and tyrosine enzymes from E. coli.
Full text
PDF


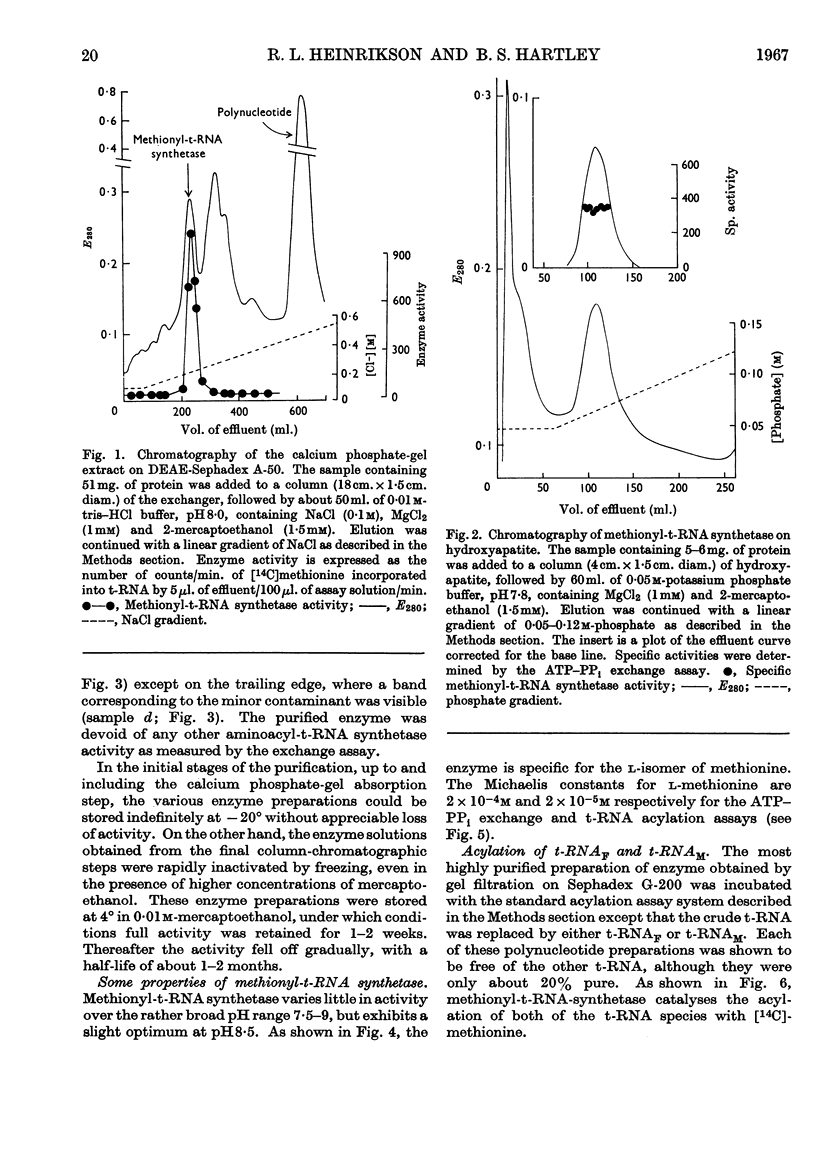
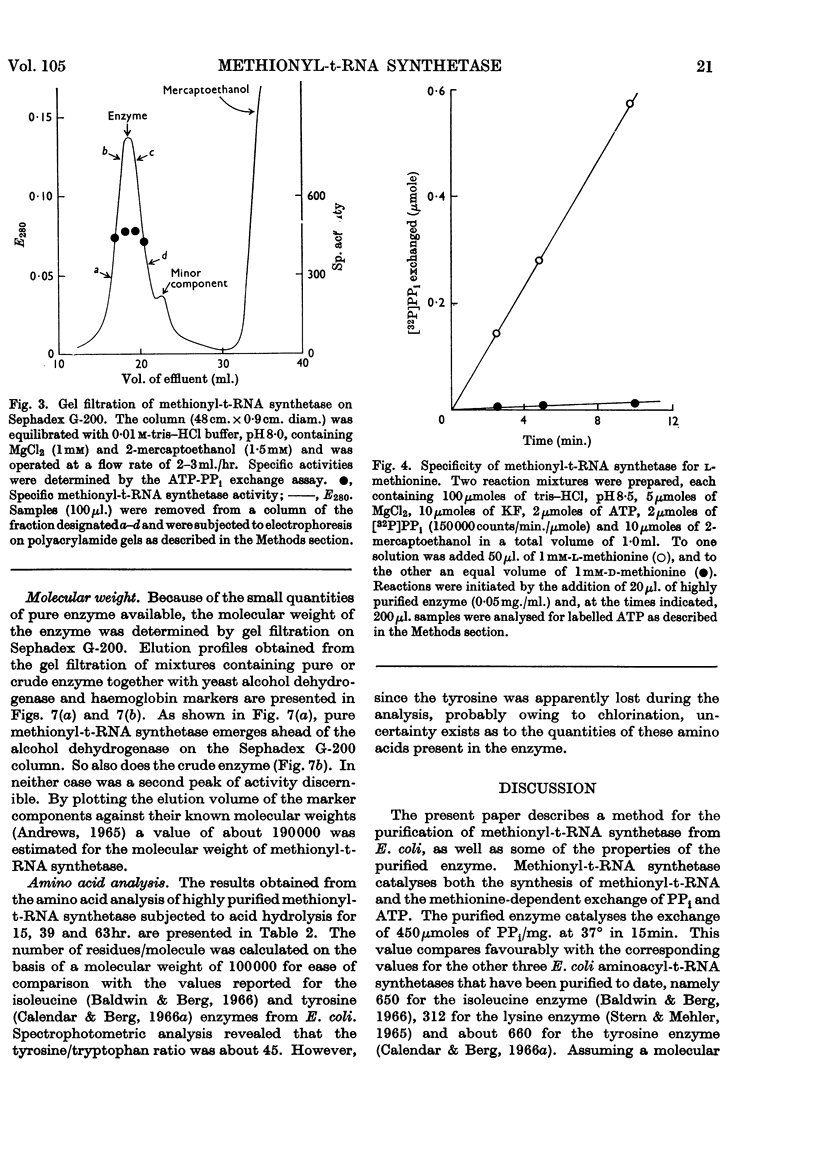
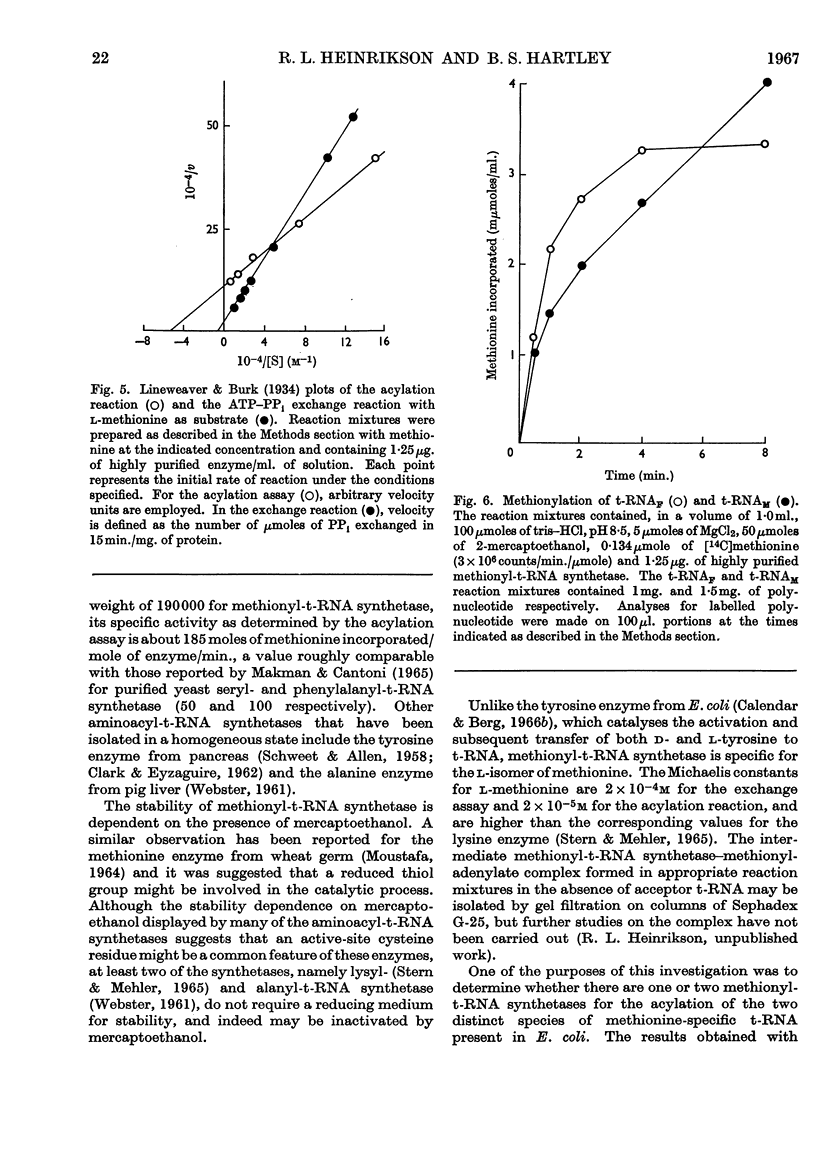
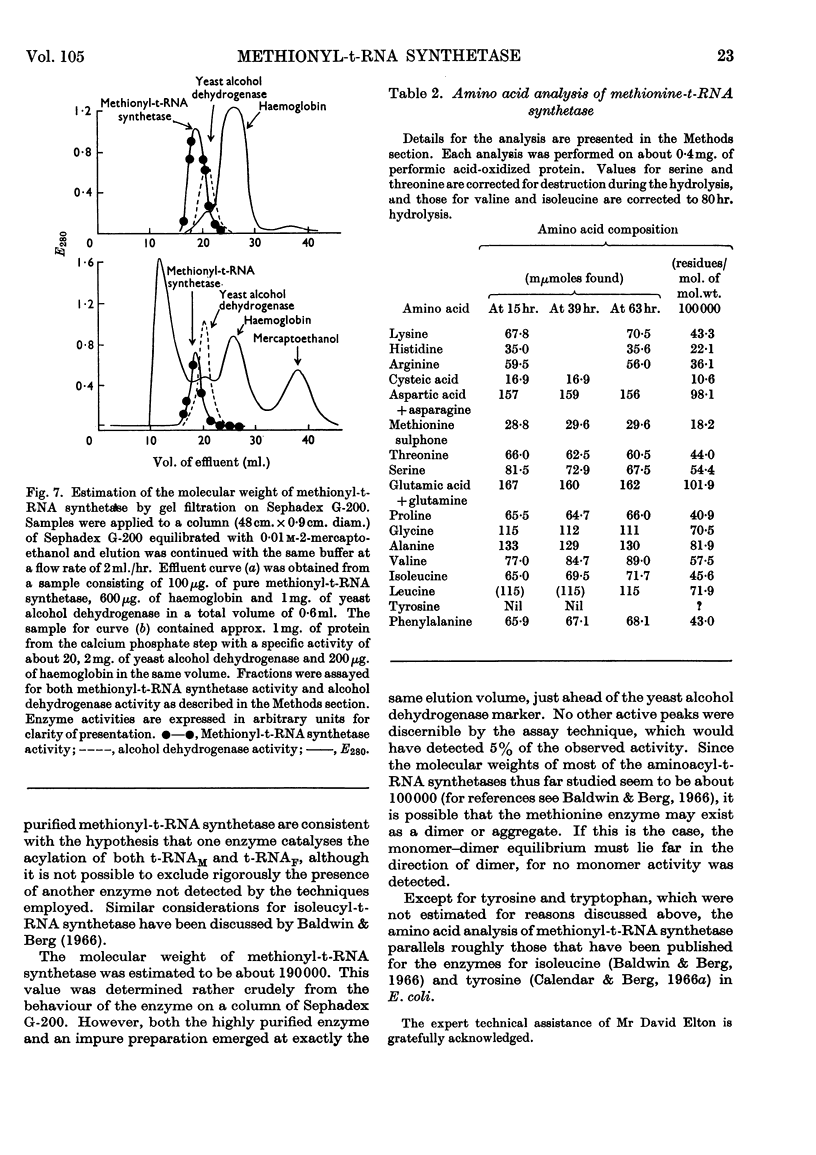

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams J. M., Capecchi M. R. N-formylmethionyl-sRNA as the initiator of protein synthesis. Proc Natl Acad Sci U S A. 1966 Jan;55(1):147–155. doi: 10.1073/pnas.55.1.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews P. The gel-filtration behaviour of proteins related to their molecular weights over a wide range. Biochem J. 1965 Sep;96(3):595–606. doi: 10.1042/bj0960595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BEAVEN G. H., HOLIDAY E. R. Ultraviolet absorption spectra of proteins and amino acids. Adv Protein Chem. 1952;7:319–386. doi: 10.1016/s0065-3233(08)60022-4. [DOI] [PubMed] [Google Scholar]
- Baldwin A. N., Berg P. Purification and properties of isoleucyl ribonucleic acid synthetase from Escherichia coli. J Biol Chem. 1966 Feb 25;241(4):831–838. [PubMed] [Google Scholar]
- CRESTFIELD A. M., STEIN W. H., MOORE S. On the preparation of bovine pancreatic ribonuclease A. J Biol Chem. 1963 Feb;238:618–621. [PubMed] [Google Scholar]
- Calendar R., Berg P. Purification and physical characterization of tyrosyl ribonucleic acid synthetases from Escherichia coli and Bacillus subtilis. Biochemistry. 1966 May;5(5):1681–1690. doi: 10.1021/bi00869a033. [DOI] [PubMed] [Google Scholar]
- HJERTEN S., LEVIN O., TISELIUS A. Protein chromatography on calcium phosphate columns. Arch Biochem Biophys. 1956 Nov;65(1):132–155. doi: 10.1016/0003-9861(56)90183-7. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MARCKER K., SANGER F. N-FORMYL-METHIONYL-S-RNA. J Mol Biol. 1964 Jun;8:835–840. doi: 10.1016/s0022-2836(64)80164-9. [DOI] [PubMed] [Google Scholar]
- MOUSTAFA E. PURIFICATION AND PROPERTIES OF LYSYL- AND METHIONYL-SOLUBLE RIBONUCLEIC ACID SYNTHETASES FROM WHEAT GERM. Biochim Biophys Acta. 1964 Nov 15;91:421–426. doi: 10.1016/0926-6550(64)90072-6. [DOI] [PubMed] [Google Scholar]
- SCHWEET R. S., ALLEN E. H. Purification and properties of tyrosine-activating enzyme of hog pancreas. J Biol Chem. 1958 Nov;233(5):1104–1108. [PubMed] [Google Scholar]
- Stern R., Mehler A. H. Lysyl-sRNA synthetase from Escherichia coli. Biochem Z. 1965 Aug 19;342(4):400–409. [PubMed] [Google Scholar]
- WEBSTER G. C. Isolation of an alanine-activating enzyme from pig liver. Biochim Biophys Acta. 1961 Apr 29;49:141–152. doi: 10.1016/0006-3002(61)90877-0. [DOI] [PubMed] [Google Scholar]
- Webster R. E., Engelhardt D. L., Zinder N. D. In vitro protein synthesis: chain initiation. Proc Natl Acad Sci U S A. 1966 Jan;55(1):155–161. doi: 10.1073/pnas.55.1.155. [DOI] [PMC free article] [PubMed] [Google Scholar]


