Abstract
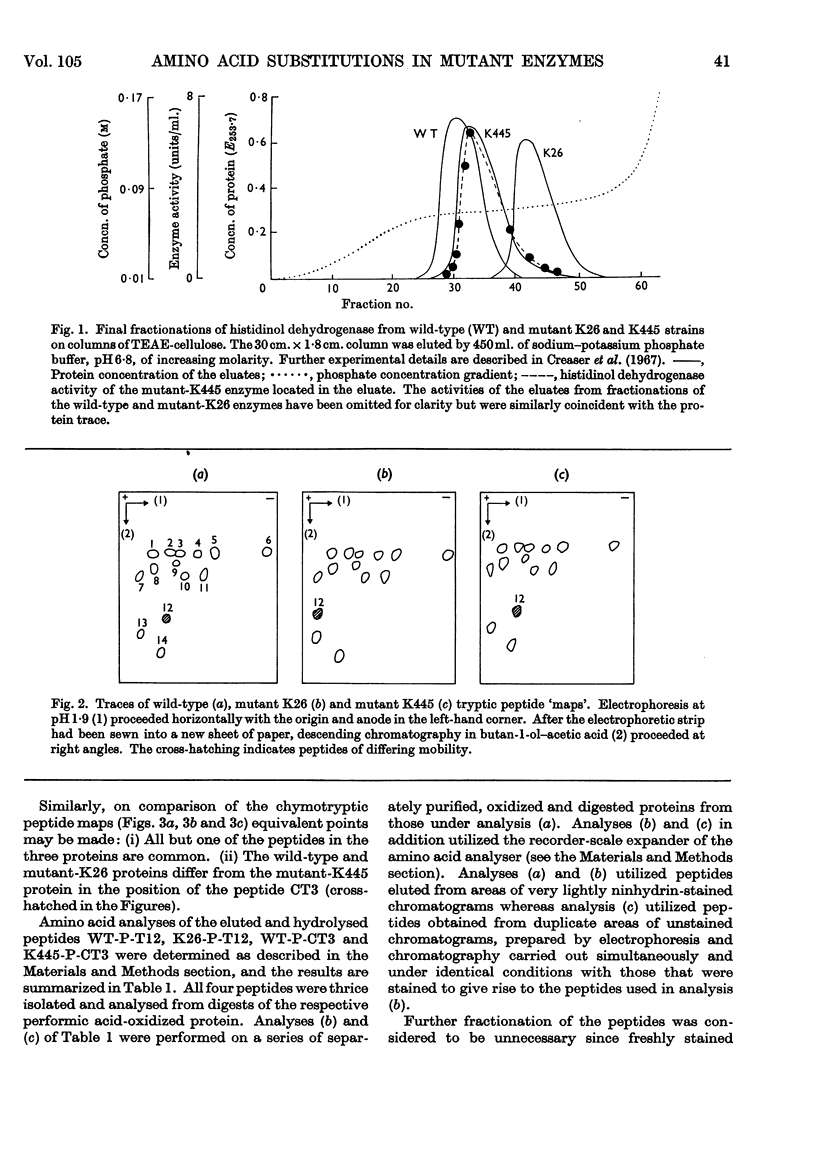
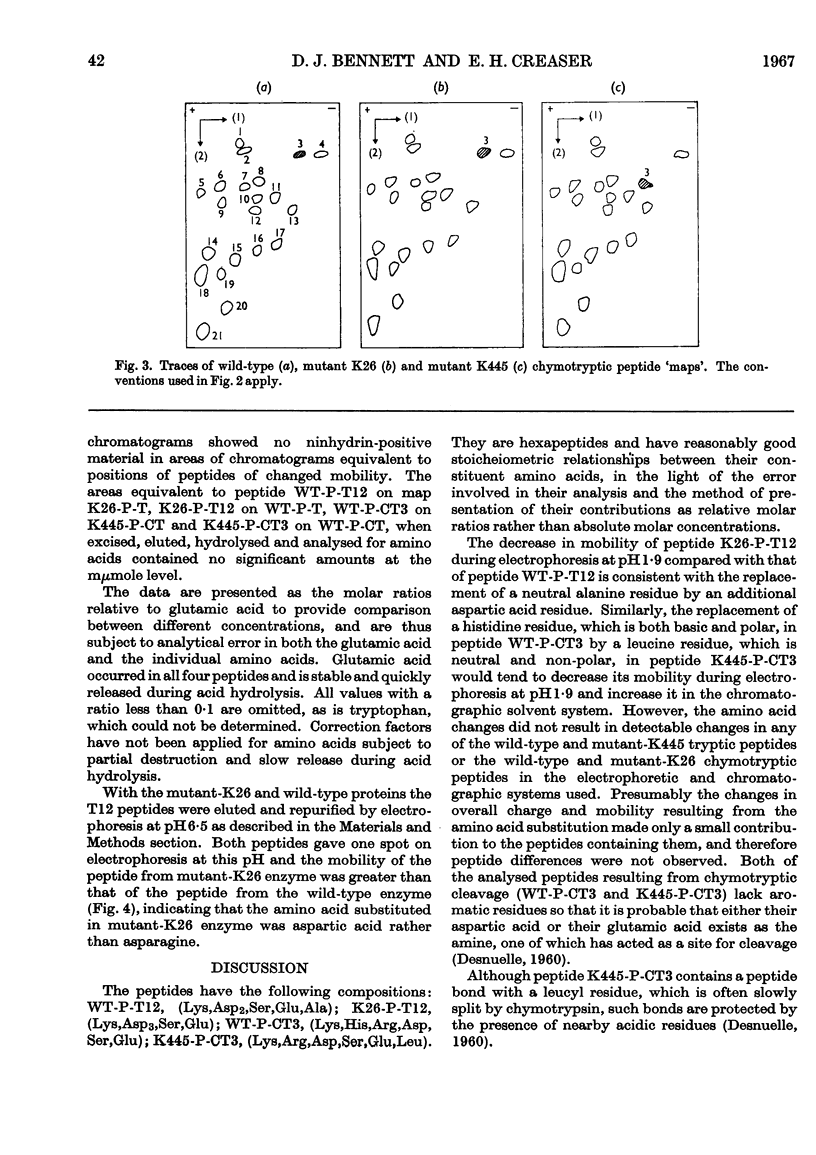
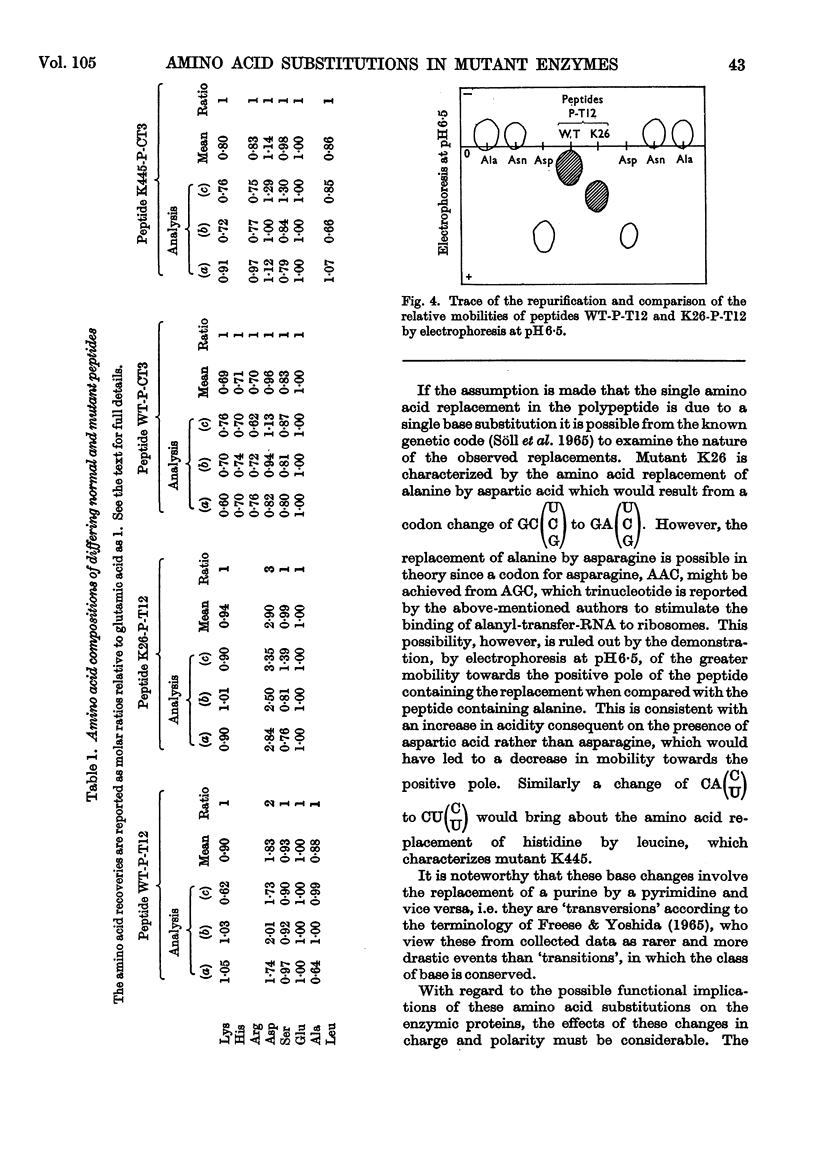
Amino acid changes in the enzyme l-histidinol dehydrogenase (l-histidinol–NAD oxidoreductase, EC 1.1.1.23) have been determined between the wild-type Neurospora crassa and two temperature-sensitive mutants. Comparison was made between amino acid analyses of peptides of differing electrophoretic and chromatographic mobilities resulting from tryptic and chymotryptic digestion of protein from wild-type and mutant K26, and wild-type and mutant K445 strains, respectively. The analyses demonstrate the substitution of aspartic acid for alanine in mutant K26, and leucine for histidine in mutant K445. The effects of the resulting changes in polarity and charge are discussed in relation to the catalytic functioning of the proteins.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- CATCHESIDE D. G. Complementation among histidine mutants of Neurospora crassa. Proc R Soc Lond B Biol Sci. 1960 Nov 29;153:179–194. doi: 10.1098/rspb.1960.0095. [DOI] [PubMed] [Google Scholar]
- Creaser E. H., Bennett D. J., Drysdale R. B. Studies on biosynthetic enzymes. I. Mutant forms of histidinol dehydrogenase from Neurospora crassa. Can J Biochem. 1965 Jul;43(7):993–1000. doi: 10.1139/o65-112. [DOI] [PubMed] [Google Scholar]
- Creaser E. H., Bennett D. J., Drysdale R. B. The purification and properties of histidinol dehydrogenase from Neurospora crassa. Biochem J. 1967 Apr;103(1):36–41. doi: 10.1042/bj1030036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EPSTEIN C. J. RELATION OF PROTEIN EVOLUTION TO TERTIARY STRUCTURE. Nature. 1964 Sep 26;203:1350–1352. doi: 10.1038/2031350a0. [DOI] [PubMed] [Google Scholar]
- MARGOLIASH E. PRIMARY STRUCTURE AND EVOLUTION OF CYTOCHROME C. Proc Natl Acad Sci U S A. 1963 Oct;50:672–679. doi: 10.1073/pnas.50.4.672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REDFIELD R. R., ANFINSEN C. B. The structure of ribonuclease. II. The preparation, separation, and relative alignment of large enzymatically produced fragments. J Biol Chem. 1956 Jul;221(1):385–404. [PubMed] [Google Scholar]
- Söll D., Ohtsuka E., Jones D. S., Lohrmann R., Hayatsu H., Nishimura S., Khorana H. G. Studies on polynucleotides, XLIX. Stimulation of the binding of aminoacyl-sRNA's to ribosomes by ribotrinucleotides and a survey of codon assignments for 20 amino acids. Proc Natl Acad Sci U S A. 1965 Nov;54(5):1378–1385. doi: 10.1073/pnas.54.5.1378. [DOI] [PMC free article] [PubMed] [Google Scholar]


