Abstract
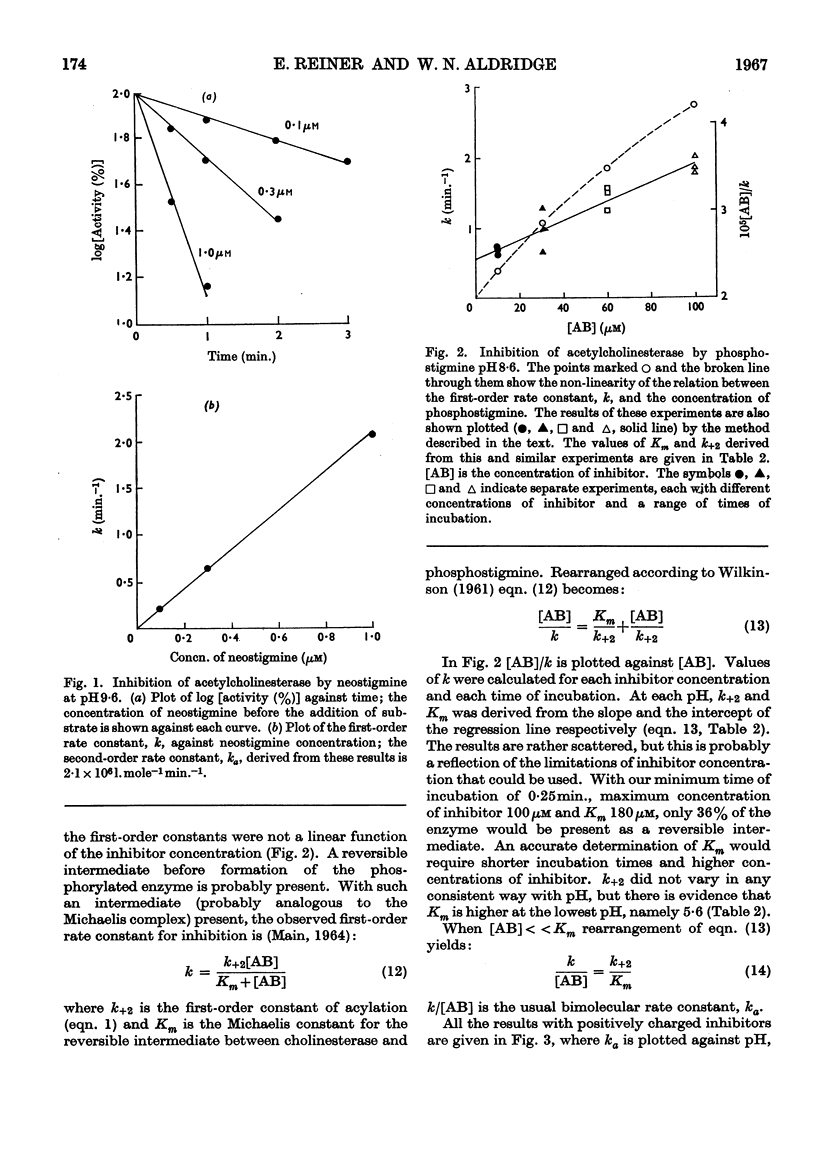
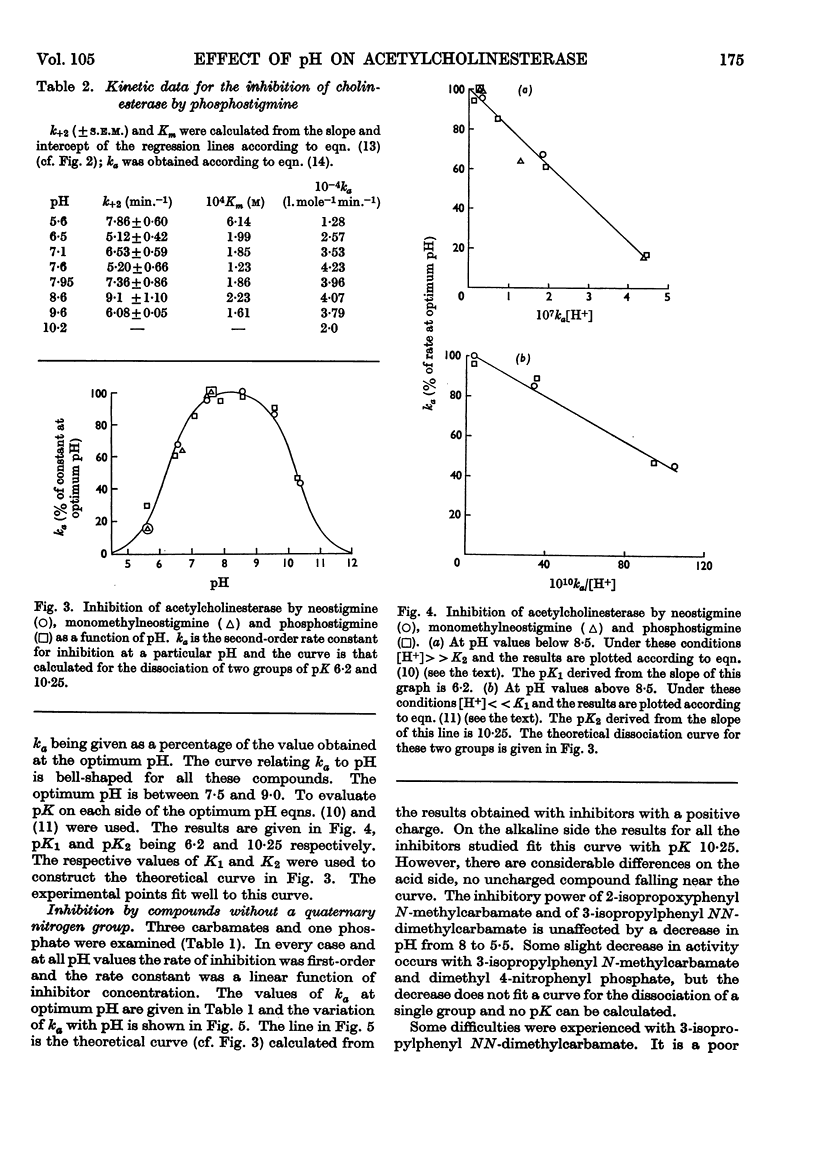
1. The second-order rate constants of inhibition, ka, of acetylcholinesterase were measured at pH values between 5·5 and 10·5 for two esters of phosphorus acids and five esters of carbamic acids. Two of the carbamates and one of the phosphates contained a quaternary nitrogen group. 2. For the three positively charged compounds the ka–pH plots are bell-shaped, with a pH optimum between 7·5 and 9·0. The changes in ka above and below the optimum pH fit theoretical curves for the dissociation of groups on the protein of pK 6·2 and 10·25. 3. For the uncharged compounds, the ka–pH plot on the alkaline side is identical with the one obtained for charged inhibitors. On the acid side they do not fit such a curve and the ka for two of the carbamates is independent of pH changes between 5·5 and 8·0. 4. The first-order rate constants, k+3, for spontaneous reactivation were measured at pH values between 5·0 and 11·0 for N-methylcarbamoylated, NN-dimethylcarbamoylated and di-(2-chloroeth)phosphorylated cholinesterase. For all three derivatives the k+3–pH plots are bell-shaped, with a pH optimum between 8·0 and 8·5. The changes in k+3 above and below the optimum fit theoretical curves for the dissociation of groups of pK 6·9 and 9·8. 5. The relevance of these results to binding, acylation and deacylation of both inhibitors and substrates is discussed.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALDRIDGE W. N., DAVISON A. N. The inhibition of erythrocyte cholinesterase by tri-esters of phosphoric acid. I. Diethyl p nitrophenyl phosphate (E600) and analogues. Biochem J. 1952 Apr;51(1):62–70. doi: 10.1042/bj0510062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ALDRIDGE W. N. [The inhibition of erythrocyte cholinesterase by tri-esters of phosphoric acid. III. The nature of the inhibitory process]. Biochem J. 1953 Jun;54(3):442–448. doi: 10.1042/bj0540442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BERGMANN F., SEGAL R., SHIMONI A., WURZEL M. The pH-dependence of enzymic ester hydrolysis. Biochem J. 1956 Aug;63(4):684–690. doi: 10.1042/bj0630684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry W. K., Davies D. R. Factors influencing the rate of "aging" of a series of alkyl methylphosphonyl-acetylcholinesterases. Biochem J. 1966 Aug;100(2):572–576. doi: 10.1042/bj1000572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coult D. B., Marsh D. J., Read G. Dealkylation studies on inhibited acetylcholinesterase. Biochem J. 1966 Mar;98(3):869–873. doi: 10.1042/bj0980869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAVISON A. N. Return of cholinesterase activity in the rat after inhibition by organophosphorus compounds. 2. A comparative study of true and pseudo cholinesterase. Biochem J. 1955 Jun;60(2):339–346. doi: 10.1042/bj0600339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ELLMAN G. L., COURTNEY K. D., ANDRES V., Jr, FEATHER-STONE R. M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol. 1961 Jul;7:88–95. doi: 10.1016/0006-2952(61)90145-9. [DOI] [PubMed] [Google Scholar]
- HEATH D. F., VANDEKAR M. Some spontaneous reactions of 00-dimethyl S-ethylthioethyl phosphorothiolate and related compounds in water and on storage, and their effects on the toxicological properties of the compounds. Biochem J. 1957 Oct;67(2):187–201. doi: 10.1042/bj0670187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HEILBRONN E. In vitro reactivation and "ageing" of Tabuninhibited blood cholinesterases; studies with N-methyl-pyridinium-2-aldoxime methane sulphonate and N,N'-trimethylene bis (pyridinium-4-aldoxime) dibromide. Biochem Pharmacol. 1963 Jan;12:25–36. doi: 10.1016/0006-2952(63)90006-6. [DOI] [PubMed] [Google Scholar]
- HOBBIGER F. The inhibition of cholinesterases by 3-(diethoxyphosphinyloxy)-N-methylquinolinium methylsulphate and its tertiary base. Br J Pharmacol Chemother. 1954 Jun;9(2):159–165. doi: 10.1111/j.1476-5381.1954.tb00835.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JANSZ H. S., BRONS D., WARRINGA M. G. Chemical nature of the DFP-binding site of pseudocholinesterase. Biochim Biophys Acta. 1959 Aug;34:573–575. doi: 10.1016/0006-3002(59)90320-8. [DOI] [PubMed] [Google Scholar]
- KITZ R., WILSON I. B. Esters of methanesulfonic acid as irreversible inhibitors of acetylcholinesterase. J Biol Chem. 1962 Oct;237:3245–3249. [PubMed] [Google Scholar]
- Krupka R. M. Hydrolysis of neutral substrates by acetylcholinesterase. Biochemistry. 1966 Jun;5(6):1983–1988. doi: 10.1021/bi00870a028. [DOI] [PubMed] [Google Scholar]
- Kunkee R. E., Zweig G. Inactivation and reactivation rates of fly and bee cholinesterases inhibited by sevin. Biochem Pharmacol. 1965 Jun;14(6):1011–1017. doi: 10.1016/0006-2952(65)90253-4. [DOI] [PubMed] [Google Scholar]
- LAMB J. C., STEINBERG G. M. COMPARISONS OF REACTIVATION AND AGING RATES OF EEL ACETYLCHOLINESTERASE INHIBITED BY GB AND 4-PPAM. Biochim Biophys Acta. 1964 Jul 8;89:171–174. doi: 10.1016/0926-6569(64)90117-8. [DOI] [PubMed] [Google Scholar]
- LAWLER H. C. Turnover time of acetylcholinesterase. J Biol Chem. 1961 Aug;236:2296–2301. [PubMed] [Google Scholar]
- LEE R. M. DI-(2-CHLOROETHYL) ARYL PHOSPHATES. A STUDY OF THEIR REACTION WITH B-ESTERASES, AND OF THE GENETIC CONTROL OF THEIR HYDROLYSIS IN SHEEP. Biochem Pharmacol. 1964 Dec;13:1551–1568. doi: 10.1016/0006-2952(64)90210-2. [DOI] [PubMed] [Google Scholar]
- MAIN A. R. AFFINITY AND PHOSPHORYLATION CONSTANTS FOR THE INHIBITION OF ESTERASES BY ORGANOPHOSPHATES. Science. 1964 May 22;144(3621):992–993. doi: 10.1126/science.144.3621.992. [DOI] [PubMed] [Google Scholar]
- MOUNTER L. A., ALEXANDER H. C., 3rd, TUCK K. D., DIEN L. T. The pH dependence and dissociation constants of esterases and proteases treated with diisopropyl fluorophosphate. J Biol Chem. 1957 Jun;226(2):867–872. [PubMed] [Google Scholar]
- MYERS D. K. Studies on cholinesterase. 10. Return of cholinesterase activity in the rat after inhibition by carbamoyl fluorides. Biochem J. 1956 Apr;62(4):556–563. doi: 10.1042/bj0620556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Main A. R., Iverson F. Measurement of the affinity and phosphorylation constants governing irreversible inhibition of cholinesterases by di-isopropyl phosphorofluoridate. Biochem J. 1966 Aug;100(2):525–531. doi: 10.1042/bj1000525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiner E., Simeon-Rudolf V. The kinetics of inhibition of erythrocyte cholinesterase by monomethylcarbamates. Biochem J. 1966 Feb;98(2):501–505. doi: 10.1042/bj0980501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WILKINSON G. N. Statistical estimations in enzyme kinetics. Biochem J. 1961 Aug;80:324–332. doi: 10.1042/bj0800324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WILSON I. B., BERGMANN F. Studies on cholinesterase. VII. The active surface of acetylcholine esterase derived from effects of pH on inhibitors. J Biol Chem. 1950 Aug;185(2):479–489. [PubMed] [Google Scholar]
- WILSON I. B., HARRISON M. A., GINSBURG S. Carbamyl derivatives of acetylcholinesterase. J Biol Chem. 1961 May;236:1498–1500. [PubMed] [Google Scholar]
- WILSON I. B., HATCH M. A., GINSBURG S. Carbamylation of acetvlcholinesterase. J Biol Chem. 1960 Aug;235:2312–2315. [PubMed] [Google Scholar]
- WILSON I. B. Mechanism of enzymic hydrolysis. I. Role of the acidic group in the esteratic site of acetylcholinesterase. Biochim Biophys Acta. 1951 Sep;7(3):466–470. doi: 10.1016/0006-3002(51)90050-9. [DOI] [PubMed] [Google Scholar]
- Winteringham F. P., Fowler K. S. Substrate and dilution effects on the inhibition of acetylcholinesterase by carbamates. Biochem J. 1966 Oct;101(1):127–134. doi: 10.1042/bj1010127. [DOI] [PMC free article] [PubMed] [Google Scholar]


