Abstract
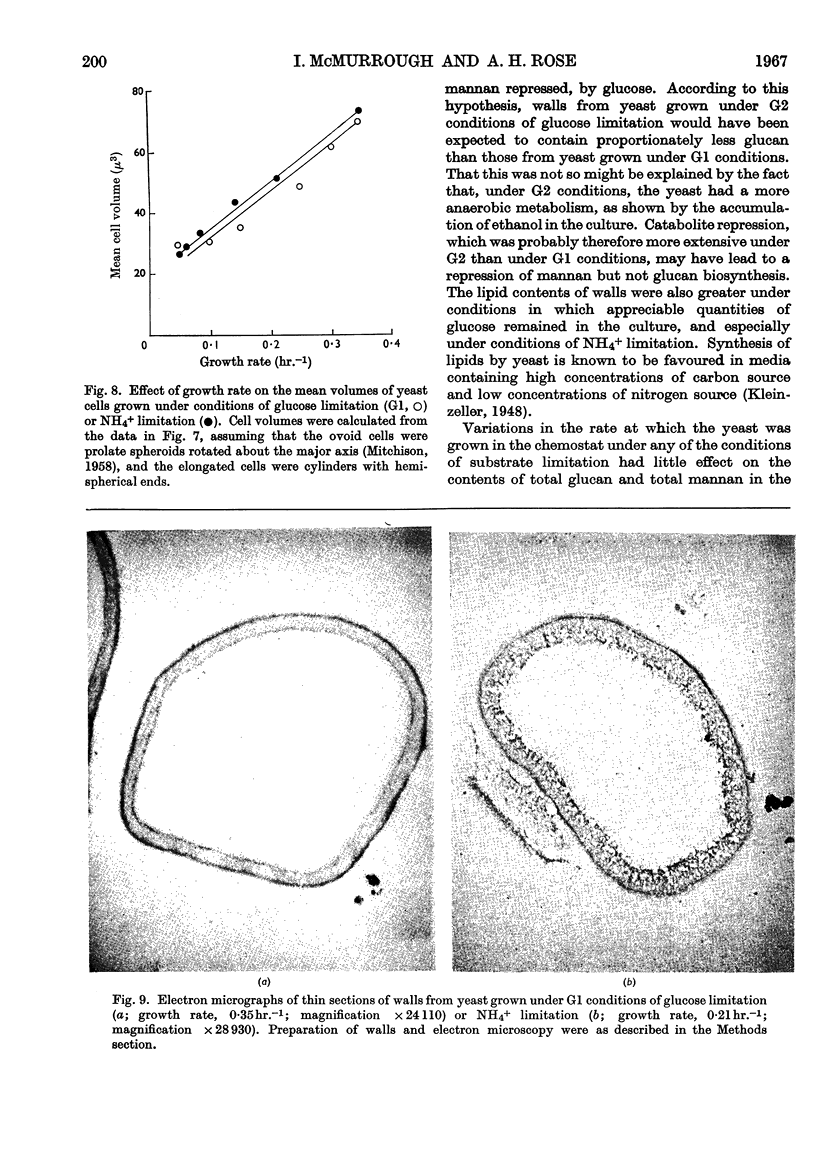
1. A study was made of the composition and structure of walls isolated from yeast grown in continuous culture at different rates, under three conditions of glucose limitation in which the concentrations of glucose and ammonium sulphate in the medium and the oxygen-transfer rate in the culture were varied, and one condition of NH4+ limitation. 2. The contents of total glucan and total mannan in the walls were relatively little affected by the growth rate under any of the four sets of conditions. The phosphorus and protein contents of walls from yeast grown under each of the four conditions increased as the growth rate was decreased. Walls from yeast grown under NH4+ limitation contained only half as much protein as walls from cells grown under glucose limitation. The proportion of lipid was greatest in walls from yeast grown under NH4+ limitation. 3. A procedure was devised for fractionating isolated walls, based on the ease with which the glucan and mannan were extracted with water and with hot and cold 6% (w/v) potassium hydroxide solution. The percentage of glucan, mannan, protein and phosphorus in each of the fractions was affected by the rate of growth and by the nature of the substrate limitation. 4. The β-fructofuranosidase activities of yeast grown under glucose limitation increased as the growth rate was lowered, but decreased at very low growth rates. The effects at low growth rates were probably due to repression of enzyme synthesis by residual glucose in the culture filtrate. The β-fructofuranosidase activities of yeast grown under NH4+ limitation were much lower than those from yeast grown under any of the conditions of glucose limitation. 5. Yeast cells grown at any of the rates under NH4+ limitation were longer and thinner than those grown at the same rate under any of the conditions of glucose limitation. Mean cell volumes were dependent on growth rate but not on the nature of the substrate limitation. 6. Electron micrographs of thin sections of isolated walls showed that cells grown under NH4+ limitation had a more porous structure than those from cells grown under any of the conditions of glucose limitation.
Full text
PDF













Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AHMAD F., ROSE A. H. The role of biotin in the regulation of enzyme synthesis in yeast. Arch Biochem Biophys. 1962 May;97:302–308. doi: 10.1016/0003-9861(62)90082-6. [DOI] [PubMed] [Google Scholar]
- BURGER M., BACON E. E., BACON J. S. Some observations on the form and location of invertase in the yeast cell. Biochem J. 1961 Mar;78:504–511. doi: 10.1042/bj0780504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown C. M., Hough J. S. Protein-disulphide reductase activity in yeast. Nature. 1966 Jul 9;211(5045):201–201. doi: 10.1038/211201a0. [DOI] [PubMed] [Google Scholar]
- DAVIES A. Invertase formation in Saccharomyces fragilis. J Gen Microbiol. 1956 Feb;14(1):109–121. doi: 10.1099/00221287-14-1-109. [DOI] [PubMed] [Google Scholar]
- DITTMER J. C., LESTER R. L. A SIMPLE, SPECIFIC SPRAY FOR THE DETECTION OF PHOSPHOLIPIDS ON THIN-LAYER CHROMATOGRAMS. J Lipid Res. 1964 Jan;5:126–127. [PubMed] [Google Scholar]
- EDDY A. A. [The structure of the yeast cell wall. II. Degradative studies with enzymes]. Proc R Soc Lond B Biol Sci. 1958 Dec 17;149(936):425–440. doi: 10.1098/rspb.1958.0085. [DOI] [PubMed] [Google Scholar]
- FALCONE G., NICKERSON W. J. Cell-wall mannan-protein of baker's yeast. Science. 1956 Aug 10;124(3215):272–273. doi: 10.1126/science.124.3215.272-a. [DOI] [PubMed] [Google Scholar]
- HANCOCK R., PARK J. T. Cell-wall synthesis by Staphylococcus aureus in the presence of chloramphenicol. Nature. 1958 Apr 12;181(4615):1050–1052. doi: 10.1038/1811050a0. [DOI] [PubMed] [Google Scholar]
- HAUSCHILD A. H., PIVNICK H. Continuous culture of Brucella abortus S.19. Can J Microbiol. 1961 Aug;7:491–505. doi: 10.1139/m61-059. [DOI] [PubMed] [Google Scholar]
- Herbert D., Phipps P. J., Tempest D. W. The chemostat: design and instrumentation. Lab Pract. 1965 Oct;14(10):1150–1161. [PubMed] [Google Scholar]
- KESSLER G., NICKERSON W. J. Glucomannan-protein complexes from cell walls of yeasts. J Biol Chem. 1959 Sep;234:2281–2285. [PubMed] [Google Scholar]
- KOLB J. J., WEIDNER M. A., TOENNIES G. Microdetermination of lipid phosphorus as a measure of bacterial membrane substance. Anal Biochem. 1963 Jan;5:78–82. doi: 10.1016/0003-2697(63)90061-7. [DOI] [PubMed] [Google Scholar]
- KORN E. D., NORTHCOTE D. H. Physical and chemical properties of polysaccharides and glycoproteins of the yeast-cell wall. Biochem J. 1960 Apr;75:12–17. doi: 10.1042/bj0750012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LINDEGREN C. C. THE FLACCID CELL WALL OF SACCHAROMYCES. Can J Genet Cytol. 1963 Sep;5:254–256. doi: 10.1139/g63-035. [DOI] [PubMed] [Google Scholar]
- LUFT J. H. Permanganate; a new fixative for electron microscopy. J Biophys Biochem Cytol. 1956 Nov 25;2(6):799–802. doi: 10.1083/jcb.2.6.799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letters R. The application of a two-dimensional paper-chromatographic technique to the analysis of phospholipids. Biochem J. 1964 Nov;93(2):313–316. doi: 10.1042/bj0930313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MANDELSTAM J., ROGERS H. J. Chloramphenicol-resistant incorporation of amino-acids into Staphylococci and cell-wall synthesis. Nature. 1958 Apr 5;181(4614):956–957. doi: 10.1038/181956a0. [DOI] [PubMed] [Google Scholar]
- MITCHISON J. M. The growth of single cells. II. Saccharomyces cerevisiae. Exp Cell Res. 1958 Aug;15(1):214–221. doi: 10.1016/0014-4827(58)90077-6. [DOI] [PubMed] [Google Scholar]
- Markham R. A steam distillation apparatus suitable for micro-Kjeldahl analysis. Biochem J. 1942 Dec;36(10-12):790–791. doi: 10.1042/bj0360790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mill P. J. Phosphomannans and other components of flocculent and non-flocculent walls of Saccharomyces cerevisiae. J Gen Microbiol. 1966 Sep;44(3):329–341. doi: 10.1099/00221287-44-3-329. [DOI] [PubMed] [Google Scholar]
- NEIDHARDT F. C. EFFECTS OF ENVIRONMENT ON THE COMPOSITION OF BACTERIAL CELLS. Annu Rev Microbiol. 1963;17:61–86. doi: 10.1146/annurev.mi.17.100163.000425. [DOI] [PubMed] [Google Scholar]
- NEIDHARDT F. C., MAGASANIK B. Studies on the role of ribonucleic acid in the growth of bacteria. Biochim Biophys Acta. 1960 Jul 29;42:99–116. doi: 10.1016/0006-3002(60)90757-5. [DOI] [PubMed] [Google Scholar]
- NICKERSON W. J. SYMPOSIUM ON BIOCHEMICAL BASES OF MORPHOGENESIS IN FUNGI. IV. MOLECULAR BASES OF FORM IN YEASTS. Bacteriol Rev. 1963 Sep;27:305–324. doi: 10.1128/br.27.3.305-324.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NORTHCOTE D. H., HORNE R. W. The chemical composition and structure of the yeast cell wall. Biochem J. 1952 May;51(2):232–236. doi: 10.1042/bj0510232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PHAFF H. J. CELL WALL OF YEASTS. Annu Rev Microbiol. 1963;17:15–30. doi: 10.1146/annurev.mi.17.100163.000311. [DOI] [PubMed] [Google Scholar]
- PREISS J. W. The localization of invertase in the yeast cell with low voltage electrons. Arch Biochem Biophys. 1958 May;75(1):186–195. doi: 10.1016/0003-9861(58)90409-0. [DOI] [PubMed] [Google Scholar]
- ROELOFSEN P. A. Yeast mannan, a cell wall constituent of baker's yeast. Biochim Biophys Acta. 1953 Mar;10(3):477–478. doi: 10.1016/0006-3002(53)90280-7. [DOI] [PubMed] [Google Scholar]
- SHOCKMAN G. D., KOLB J. J., TOENNIES G. Relations between bacterial cell wall synthesis, growth phase, and autolysis. J Biol Chem. 1958 Feb;230(2):961–977. [PubMed] [Google Scholar]
- SUD I. J., SCHAECHTER M. DEPENDENCE OF THE CONTENT OF CELL ENVELOPES ON THE GROWTH RATE OF BACILLUS MEGATERIUM. J Bacteriol. 1964 Dec;88:1612–1617. doi: 10.1128/jb.88.6.1612-1617.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SYKES J., TEMPEST D. W. THE EFFECT OF MAGNESIUM AND OF CARBON LIMITATION ON THE MACROMOLECULAR ORGANISATION AND METABOLIC ACTIVITY OF PSEUDOMONAS SP., STRAIN C-IB. Biochim Biophys Acta. 1965 May 11;103:93–108. doi: 10.1016/0005-2787(65)90543-5. [DOI] [PubMed] [Google Scholar]
- Skipski V. P., Peterson R. F., Barclay M. Quantitative analysis of phospholipids by thin-layer chromatography. Biochem J. 1964 Feb;90(2):374–378. doi: 10.1042/bj0900374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VITOLS E., NORTH R. J., LINNANE A. W. Studies on the oxidative metabolism of Saccharomyces cerevisiae. I. Observations on the fine structure of the yeast cell. J Biophys Biochem Cytol. 1961 Mar;9:689–699. doi: 10.1083/jcb.9.3.689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WADE H. E., MORGAN D. M. The nature of the fluctuating ribonucleic acid in Escherichia coli. Biochem J. 1957 Feb;65(2):321–331. doi: 10.1042/bj0650321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WOOD P., IMAICHI K., KNOWLES J., MICHAELS G., KINSELL L. THE LIPID COMPOSITION OF HUMAN PLASMA CHYLOMICRONS. J Lipid Res. 1964 Apr;5:225–231. [PubMed] [Google Scholar]
- Weimberg R., Orton W. L. Elution of exocellular enzymes from Saccharomyces fragilis and Saccharomyces cerevisiae. J Bacteriol. 1966 Jan;91(1):1–13. doi: 10.1128/jb.91.1.1-13.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]



