Abstract
1. The stability of monolayers of a highly unsaturated yeast lecithin labelled with 32P has been investigated by a surface radioactivity technique. 2. Lecithin films on distilled water at all surface pressures between 6 and 48dynes/cm. were completely stable on rapid perfusion of the subphase and on addition of ionic amphipathic substances to the film. 3. Ultrasonically treated lecithin added to the subphase caused a slow loss of surface radioactivity but little pressure change. 4. The addition of proteins to the subphase caused negligible changes in the film even when conditions were favourable for electrostatic heterocoagulation and penetration. 5. Lecithin films were not hydrolysed by a strongly acid subphase at room temperature. The very low rate of hydrolysis produced by alkali was proportional to the subphase OH−ion concentration: the apparent activation energy and temperature coefficient (Q10) of the reaction were 14250 cal. and 2·37 respectively. 6. Alkaline hydrolysis of lecithin monolayers was markedly stimulated by adding methanol (10–20%, v/v) to the subphase. The addition of ionic amphipaths to the monolayer had the expected type of effect on the hydrolysis rate, but its magnitude was far less than that suggested by an application of the Poisson–Boltzmann equation for ion distribution at a charged interface (Davies & Rideal, 1963).
Full text
PDF
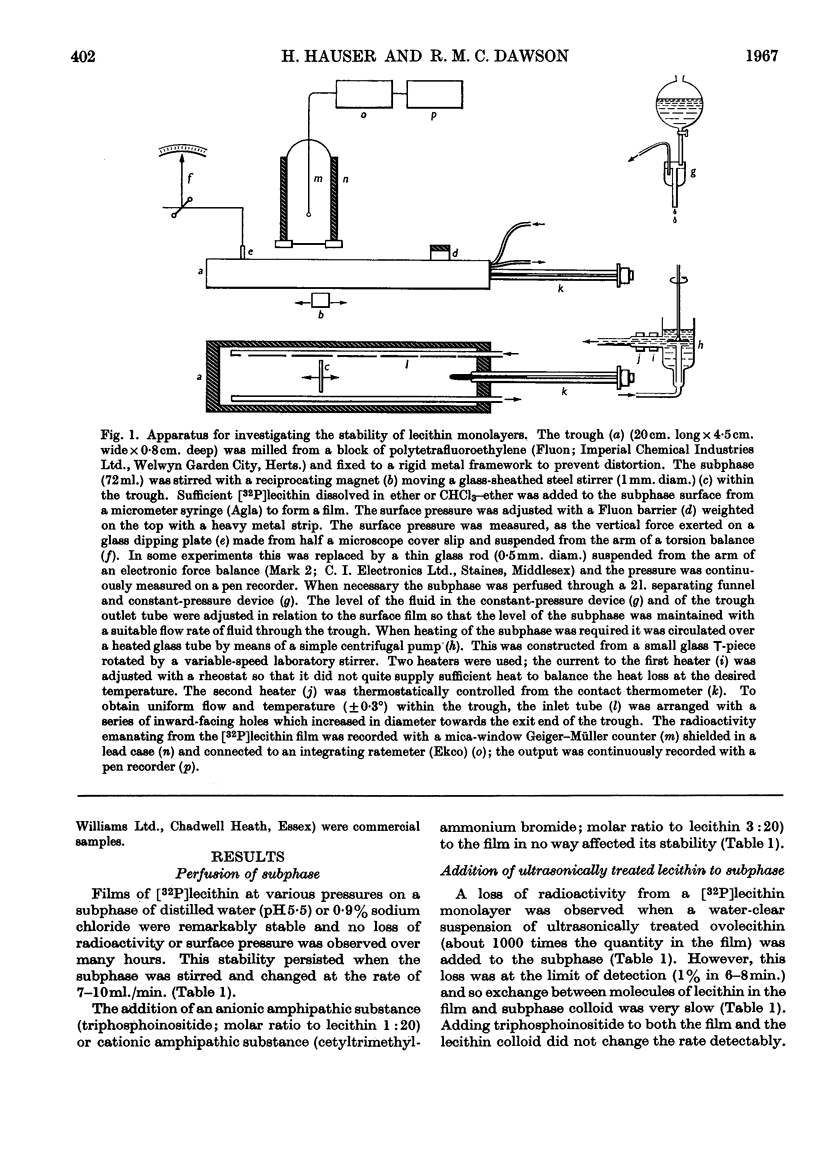
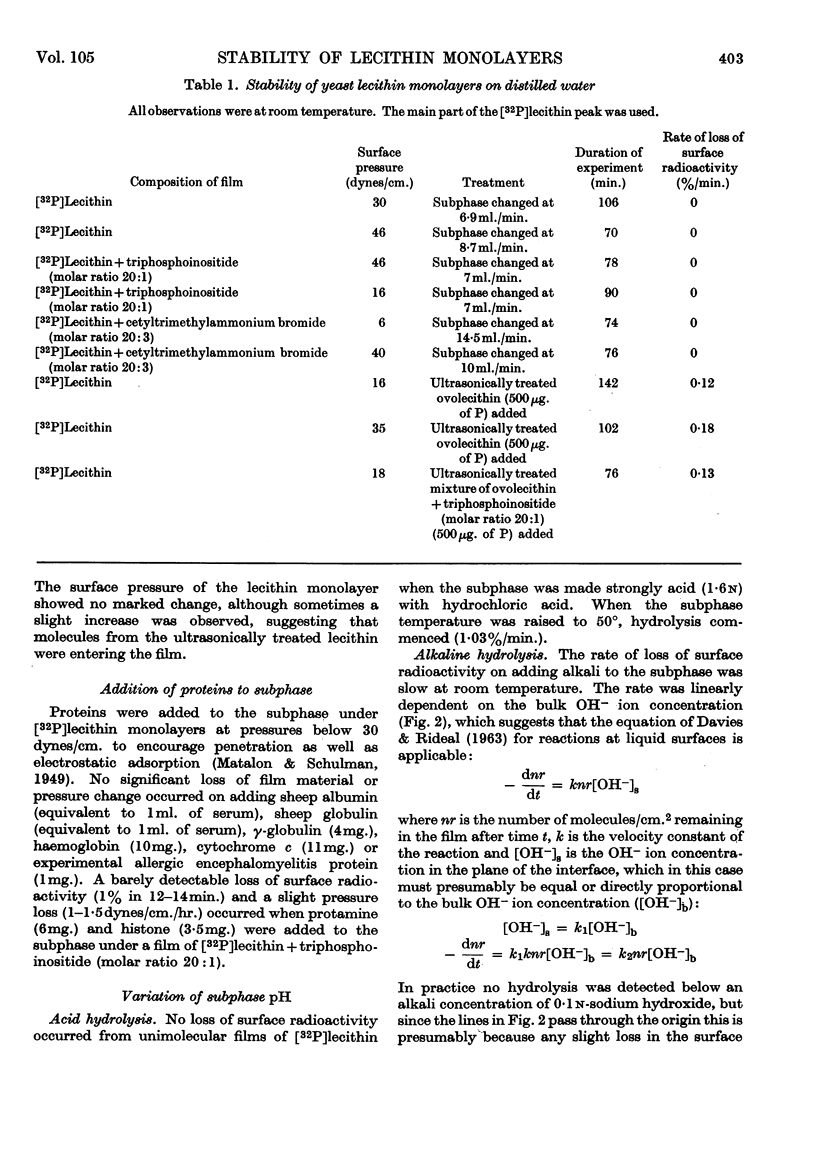
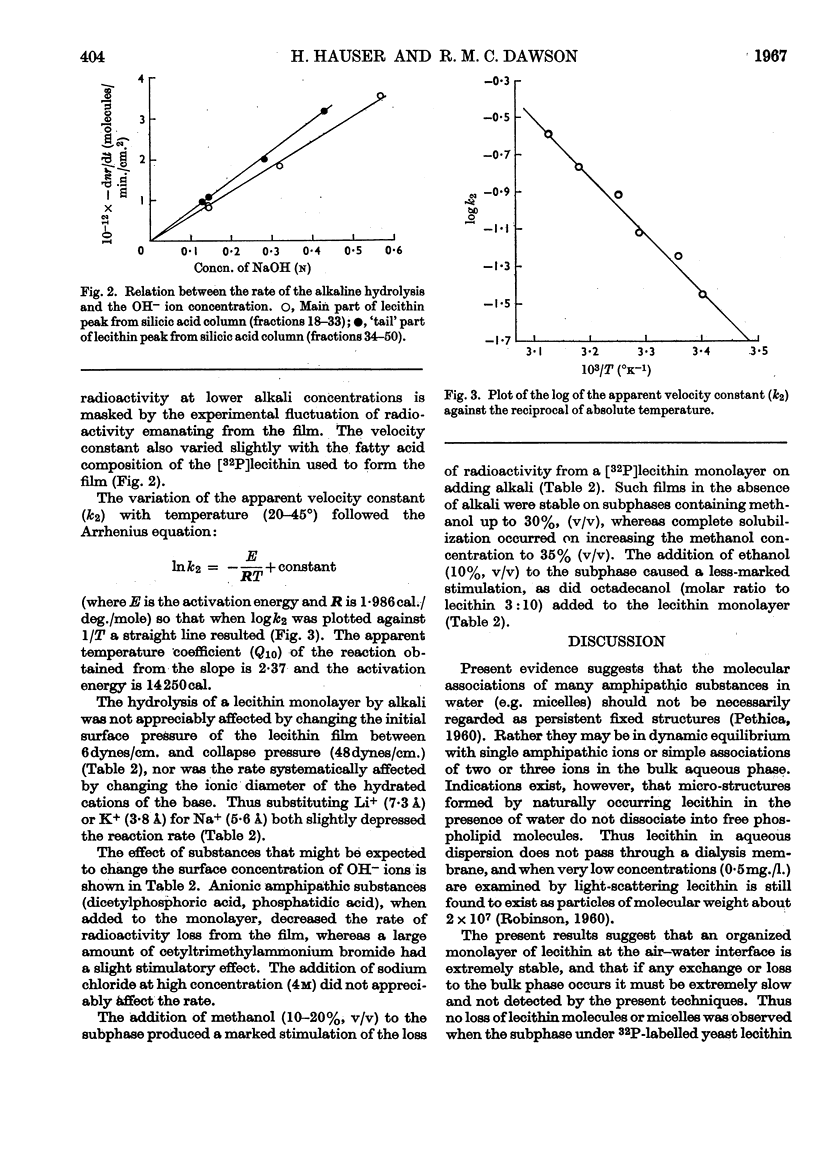
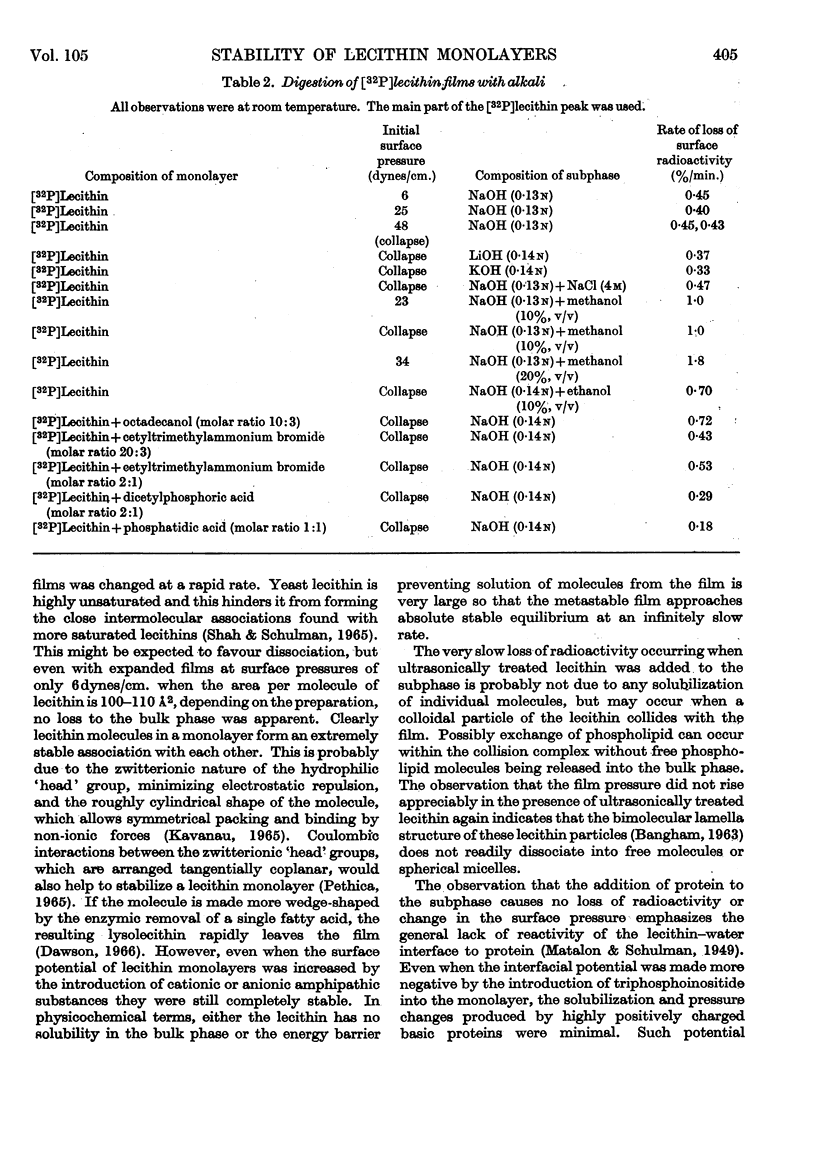


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BANGHAM A. D., DAWSON R. M. The physicochemical requirements for the action of Penicillium notatum phospholipase B on unimolecular films of lecithin. Biochem J. 1960 Apr;75:133–138. doi: 10.1042/bj0750133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BANGHAM A. D., DAWSON R. M. The relation between the activity of a lecithinase and the electrophoretic charge of the substrate. Biochem J. 1959 Jul;72:486–492. doi: 10.1042/bj0720486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BANGHAM A. D. PHYSICAL STRUCTURE AND BEHAVIOR OF LIPIDS AND LIPID ENZYMES. Adv Lipid Res. 1963;1:65–104. doi: 10.1016/b978-1-4831-9937-5.50008-9. [DOI] [PubMed] [Google Scholar]
- DITTMER J. C., DAWSON R. M. The isolation of a new lipid, triphosphoinositide, and monophosphoinositide from ox brain. Biochem J. 1961 Dec;81:535–540. doi: 10.1042/bj0810535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson R. M., Hemington N. Some properties of purified phospholipase D and especially the effect of amphipathic substances. Biochem J. 1967 Jan;102(1):76–86. doi: 10.1042/bj1020076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARINETTI G. V. Hydrolysis of lecithin with sodium methoxide. Biochemistry. 1962 Mar;1:350–353. doi: 10.1021/bi00908a024. [DOI] [PubMed] [Google Scholar]
- SHAH D. O., SCHULMAN J. H. BINDING OF METAL IONS TO MONOLAYERS OF LECITHINS, PLASMALOGEN, CARDIOLIPIN, AND DICETYL PHOSPHATE. J Lipid Res. 1965 Jul;6:341–349. [PubMed] [Google Scholar]


