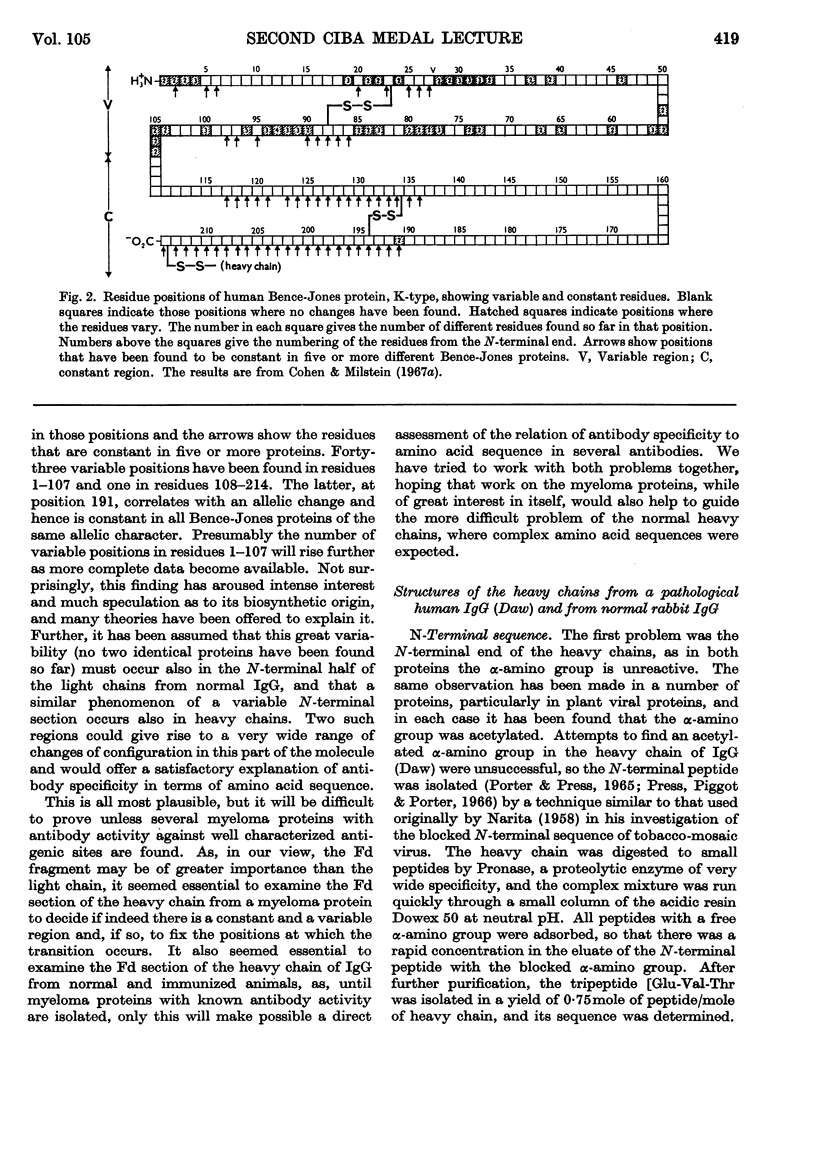
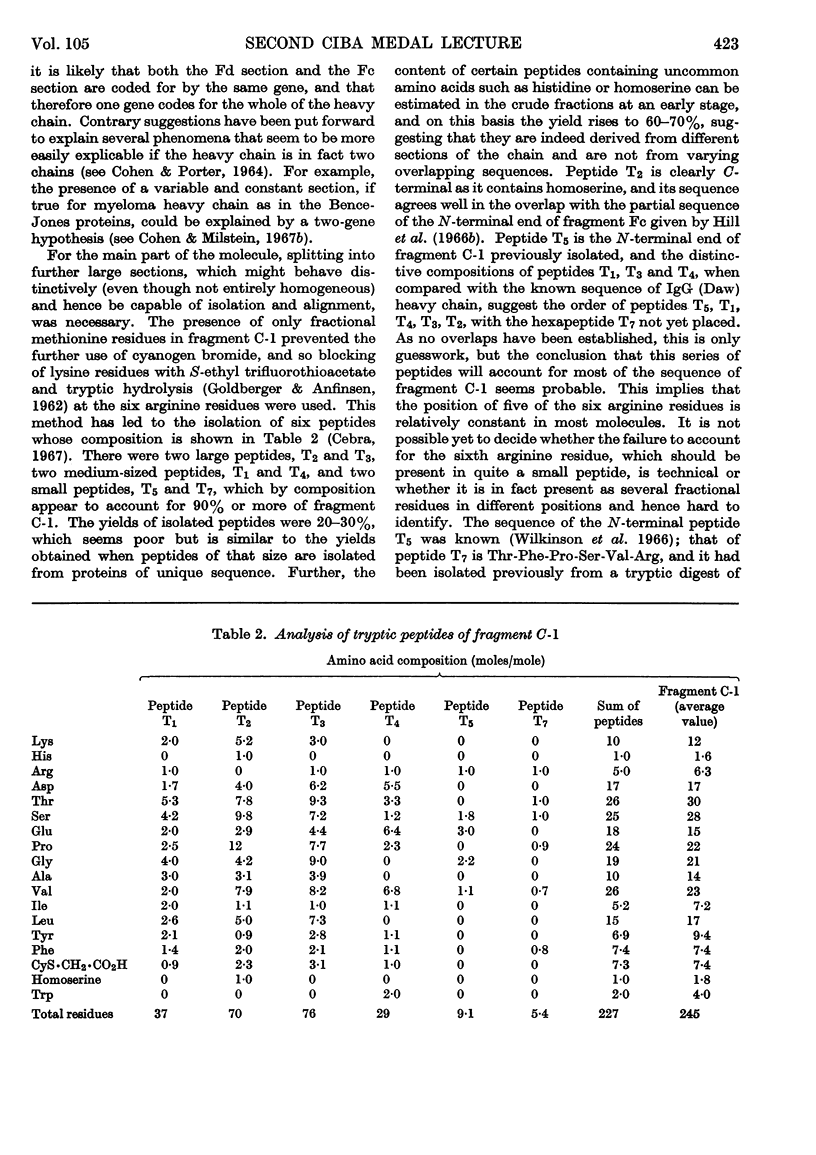
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- COHEN S., PORTER R. B. STRUCTURE AND BIOLOGICAL ACTIVITY OF IMMUNOGLOBULINS. Adv Immunol. 1964;27:287–349. doi: 10.1016/s0065-2776(08)60710-5. [DOI] [PubMed] [Google Scholar]
- Cohen S., Milstein C. Structure of antibody molecules. Nature. 1967 Apr 29;214(5087):449–passim. doi: 10.1038/214449a0. [DOI] [PubMed] [Google Scholar]
- Fleischman J. B. Immunoglobulins. Annu Rev Biochem. 1966;35:835–872. doi: 10.1146/annurev.bi.35.070166.004155. [DOI] [PubMed] [Google Scholar]
- Gray W. R., Dreyer W. J., Hood L. Mechanism of antibody synthesis: size differences between mouse kappa chains. Science. 1967 Jan 27;155(3761):465–467. doi: 10.1126/science.155.3761.465. [DOI] [PubMed] [Google Scholar]
- HABER E. RECOVERY OF ANTIGENIC SPECIFICITY AFTER DENATURATION AND COMPLETE REDUCTION OF DISULFIDES IN A PAPAIN FRAGMENT OF ANTIBODY. Proc Natl Acad Sci U S A. 1964 Oct;52:1099–1106. doi: 10.1073/pnas.52.4.1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill R. L., Delaney R., Fellows R. E., Lebovitz H. E. The evolutionary origins of the immunoglobulins. Proc Natl Acad Sci U S A. 1966 Dec;56(6):1762–1769. doi: 10.1073/pnas.56.6.1762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milstein C., Clegg J. B., Jarvis J. M. C-terminal half of immunoglobulin lambda-chains. Nature. 1967 Apr 15;214(5085):270–272. doi: 10.1038/214270a0. [DOI] [PubMed] [Google Scholar]
- NARITA K. Isolation of acetylpeptide from enzymic digests of TMV-protein. Biochim Biophys Acta. 1958 Apr;28(1):184–191. doi: 10.1016/0006-3002(58)90445-1. [DOI] [PubMed] [Google Scholar]
- Piggot P. J., Press E. M. Cyanogen bromide cleavage and partial sequence of the heavy chain of a pathological immunoglobulin G. Biochem J. 1967 Aug;104(2):616–626. doi: 10.1042/bj1040616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prahl J. W. Enzymic degradation of the Fc fragment of rabbit immunoglobulin IgG. Biochem J. 1967 Aug;104(2):647–655. doi: 10.1042/bj1040647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Press E. M., Piggot P. J., Porter R. R. The N- and c-terminal amino acid sequences of the heavy chain from a pathological human immunoglobulin IgG. Biochem J. 1966 May;99(2):356–366. doi: 10.1042/bj0990356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STEMKE G. W. ALLOTYPIC SPECIFICITIES OF A- AND B-CHAINS OF RABBIT GAMMA GLOBULIN. Science. 1964 Jul 24;145(3630):403–405. doi: 10.1126/science.145.3630.403. [DOI] [PubMed] [Google Scholar]
- Sie H. G., Hablanian A. Depletion of glycogen synthetase and increase of glucose 6-phosphate dehydrogenase in livers of ethionine-treated mice. Biochem J. 1965 Oct;97(1):32–36. doi: 10.1042/bj0970032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Titani K., Wikler M., Putnam F. W. Evolution of immunoglobulins: structural homology of kappa and lambda Bence Jones proteins. Science. 1967 Feb 17;155(3764):828–835. doi: 10.1126/science.155.3764.828. [DOI] [PubMed] [Google Scholar]
- WHITNEY P. L., TANFORD C. RECOVERY OF SPECIFIC ACTIVITY AFTER COMPLETE UNFOLDING AND REDUCTION OF AN ANTIBODY FRAGMENT. Proc Natl Acad Sci U S A. 1965 Mar;53:524–532. doi: 10.1073/pnas.53.3.524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weir R. C., Porter R. R. The antigen-binding capacity of the peptide chains of horse antibodies. Biochem J. 1966 Jul;100(1):69–72. doi: 10.1042/bj1000069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson J. M., Press E. M., Porter R. R. The N-terminal sequence of the heavy chain of rabbit immunoglobulin IgG. Biochem J. 1966 Aug;100(2):303–308. doi: 10.1042/bj1000303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zettervall O., Sjöquist J., Waldenström J., Winblad S. Serological activity in myeloma type golbulins. Clin Exp Immunol. 1966 Apr;1(2):213–222. [PMC free article] [PubMed] [Google Scholar]



