ABSTRACT
Human brain imaging took off in the 1980s and has since flooded the zone in the analysis of gender differences in behavior and mental health. Couched in the aims of “precision medicine,” the vast majority of this research has taken a binary approach, dividing participants according to the M/F box at intake and asserting that the sex differences found in neuroimaging will lead to important advances for treating neuropsychiatric disorders. However, the actual findings from this 40‐year project have not lived up to its promise, in part because of the over‐binarization of sex and general ignorance of gender as a complex variable influencing human behavior and brain function. This paper reviews the history of failed claims about male–female brain difference in the modern era, illuminates the deep‐pocketed incentives driving such research, and examines the limitations of this binary approach for understanding gender‐related behavior and health disparities. It then considers more recent efforts to “break the binary” by using measures of “gender” in addition to “sex” as an independent variable in brain imaging studies. Given the multidimensional nature of gender—as identity, expression, roles and relations—this is challenging to implement, with initial efforts producing little of substance. Better approaches to addressing male–female disparities in brain health will require focusing on specific behaviors (e.g., anxiety, risk‐taking, verbal memory, spatial navigation) and specific components of sex and gender (e.g., body size, hormone levels, gene expression, caregiver role, financial independence, discrimination) when seeking brain‐behavior correlates in a diverse population.
Keywords: gender essentialism, machine learning, mental health, MRI, neuroimaging, neurosexism, sexual dimorphism
1. Introduction
The study of sex differences in the brain has a long and sordid history. Resting on scholarly arguments for male supremacy stretching back to Aristotle, it reached its heyday in the late 19th century. In the wake of Charles Darwin's theory and own hierarchical views about humanity (Fuentes 2021), the renowned French anatomist, Paul Broca, painstakingly weighed cadaver brains and discovered what came to be known as the “missing five ounces of the female brain” widely seized upon as evidence of women's inferior minds. In 1879, this number was cited to declare that women “represent the lowest forms of human evolution” by Gustave Le Bon, the French anthropologist whom Stephen Jay Gould (1980) called “chief misogynist of Broca's school.”
Such sentiments lingered well into the 20th century, eventually giving way to a more benevolent form of sexism (Glick and Fiske 1997) that continues to shape modern neuroscience (Halpern 2010). With the dawn of magnetic resonance imaging (MRI) in the 1980s came a new way of exploring brain sex differences and a promise to find the “real” basis for well‐known, albeit often stereotyped, psychological differences between men and women. Central to this project was the assertion that different ≠ unequal (Cahill 2014): that women and men are different, but “complementary” (Gur and Gur 2017), such that neither sex beats the other in the tally of psychological strengths. With any equity concerns thus dispatched, researchers eagerly set out to find sex differences in the brain that would explain familiar patterns of psychological attributes and mental health in men versus women.
Nonetheless, the research has remained a product of its time, producing a string of high‐visibility studies claiming brain sex differences that conspicuously align with conventional gender roles. Such imaging studies took off in the 1990s, the same era when the field of evolutionary psychology was launched (e.g., Barkow et al. 1992) and entered the public imagination through popularizing works such as Men are From Mars, Women are From Venus (Gray 1992). The prevailing view remains that evolution—acting through genes and gonadal hormones—has led to fundamental, fixed differences between the brains of men and women that explain gender differences in sex drive, career preference, and relational styles, among other behaviors (DeCasien et al. 2022; Murray 2020; Stewart‐Williams and Halsey 2021). So while the overt misogyny has been eliminated, the modern study of brain sex differences remains largely preoccupied with explaining binary gender stereotypes, and is accordingly limited in what it adds to our understanding of actual behavioral diversity.
Beginning with the various incentives driving brain sex difference research, this paper will review a succession of erroneous claims propagated by the field in this modern era, demonstrating that its promise of supporting “precision” or “personalized” medicine has not been fulfilled. It will then turn to the study of gender as an independent variable in brain imaging research and recent attempts to bring a non‐binary approach to understanding behavioral and health disparities. While more laudable in its goals, this gender‐based approach is a complex project that has yet to bear significant fruit. Despite widespread advocacy for studying sex and gender differences in the brain, the existing large body of data already demonstrates that this approach is not adding meaningfully to our understanding of the neural basis of behavior and mental disorders across diverse human populations.
2. Terms and Conditions
The terms “sex” and “gender” have undergone much‐needed redefinition in recent years, based on the recognition that each is multifactorial and the two variables are intimately intertwined. For the purposes of this paper, I favor the definitions provided by a consensus report for biomedical researchers (US National Academies of Sciences, Engineering, and Medicine 2022) that defines “sex” as:
A multidimensional construct based on a cluster of anatomical and physiological traits, that include external genitalia, secondary sex characteristics, gonads, chromosomes, and hormones.
The same report defines “gender” as:
A social and cultural variable that encompasses several domains, each of which influences health: gender identity and expression, gender roles and norms, gender relations, structural sexism, power, and equality and equity.
Note that neither variable is binary and there is bidirectional influence between them. Sex characteristics influence gender identity, expression, roles, and so forth through both biological and social mechanisms. Less familiar is the fact that gender attributes also influence sex characteristics, ranging from elective genital surgery for gender affirmation to the social titration of gonadal hormone levels throughout the lifespan (van Anders and Watson 2006). Some researchers therefore favor the conjoined terms “sex/gender” or “gender/sex” when conducting or discussing any research in this area (Hyde et al. 2019). However, since the vast majority of research on human male/female brain difference has taken a binary approach and refers to its findings as “sex differences” I adopt the same terminology here, especially since it allows me to contrast such research to more recent attempts to stratify neuroimaging participants by non‐binary gender attributes. When referring strictly to behavioral differences or health disparities, I will adhere to the more commonly used term “gender,” even though such differences are likely shaped by some entanglement of the various sex‐ and gender‐related factors defined above. Finally, I use the conjoined term “gender/sex” when referring to research that may be taking account of either variable, or that is explicitly considering both variables in some combination.
When it comes to male/female differences in the brain, behavior, and mental health, the multiple factors comprising gender/sex traverse many levels of analysis, from molecular (genes, hormones) to physical (head size and shape, lean body mass, activity level, nutrition, longevity) to sociocultural (job status, financial security, caregiver burden, support network, discrimination and abuse). As we will see, even simple differences in brain structure require analysis at all of these levels, and the complexity of causation only rises when considering actual behavior and psychiatric conditions.
3. Explosion in Studies of Brain Sex Differences
Invented by Lauterbur (1973), MRI took about a decade to be applied to studying human brain sex difference. At last check, a PubMed search for “human and brain and MRI and (sex or gender)” revealed 18 669 publications dating back to 1983. The number of studies rose slowly in the 1980s but the rise became exponential through the 1990s and early 2000s, leveling out to over 1400 papers annually over the last half‐decade (Figure 1). This rise is far steeper than the roughly tripling of total PubMed articles indexed annually over the same 42‐year period.
FIGURE 1.
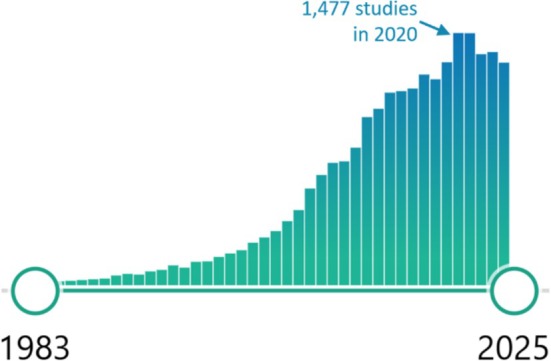
Number of papers published each year that are captured by the search terms “human and brain and MRI and (sex or gender)” on the PubMed database from the US National Library of Medicine. Searched fields include article and journal titles, abstracts, keywords, and medical subject headings (MeSH terms).
There are many reasons for the enormous focus on human brain sex differences, chief among them the fascination with behavioral differences between men and women that captivate scientists and lay people alike (Eliot 2011; Maney 2015). Put simply, sex differences are sexy, and often garner outsized attention from university press officers and science journalists who feature such findings in popular media (Fine 2010), giving neuroscientists added incentive to look for them.
A loftier rationale for studying human brain sex differences is the well‐known disparities in several common neurobehavioral disorders. These include: autism, ADHD, depression, dyslexia, substance use disorders, and Parkinson's disease, all of which are more common in men; and anxiety, depression, eating disorders, dementia, and multiple sclerosis, all more common in women (Figure 2). Although the etiologies of such differences are generally recognized to involve a combination of both sex‐ and gender‐based factors, these striking male/female prevalence ratios are often invoked in grant applications and publications to justify the search for binary sex differences in human brain structure and function.
FIGURE 2.
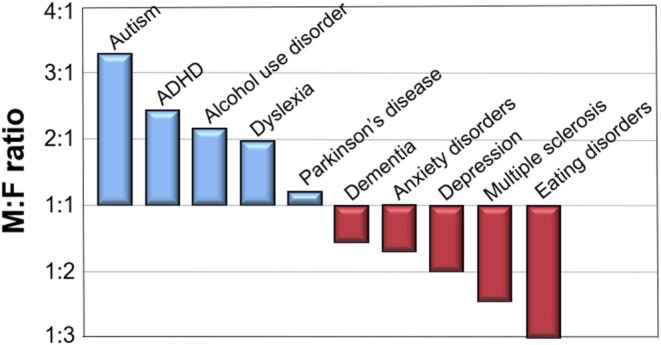
Gender prevalence ratios for 10 major neurobehavioral disorders. Sources: autism (Loomes, Hull, and Mandy 2017); ADHD (Polanczyk et al. 2007); alcohol use disorder (Grant et al. 2004); dyslexia (Rutter et al. 2004); Parkinson disease (Zirra et al. 2023); dementia (Buckley et al. 2019); anxiety disorders (McLean et al. 2011); depression (Salk et al. 2017); multiple sclerosis (Briggs and Hill 2020); eating disorders (Hudson et al. 2007).
On a more practical level are various institutional incentives that have fueled the rise of brain sex difference research. Although human brain imaging predates the 1993 National Institutes of Health (NIH) Revitalization Act, one can see a surge in studies around this time when the US Congress first mandated the inclusion of women and underrepresented minorities in federally funded human research. Now researchers who may not have been interested in gender/sex were suddenly in possession of data that could be easily mined for additional analyses and publications. The pattern repeats any time a new brain imaging method is developed and gets applied to sex differences, often with great fanfare (O'Connor and Joffe 2014), as seen by the progression of high‐profile sex difference studies using BOLD‐fMRI (Shaywitz et al. 1995), followed by resting‐state functional connectivity (rsFC) (Biswal et al. 2010), diffusion tensor imaging (DTI) (Ingalhalikar et al. 2014), and the many variations on multivariate machine learning (ML) (Sepehrband et al. 2018; Shanmugan et al. 2022; Ryali et al. 2024).
Most recently, a surge in sex difference studies has been spurred by the women's health movement, an international effort to elevate the study of women and female animals with the aim of reducing aforementioned gender health disparities (Miller et al. 2015; Ferretti et al. 2018). Whereas sex inclusion had become the norm for research on humans, male‐only studies of animals were still predominant in many biomedical research areas, most notably in neuroscience (Beery and Zucker 2011). In response, over the last decade several national and international health agencies have explicitly added policies to require sex and/or gender analyses by all grant recipients (White et al. 2021), whether studying animal or human subjects. For the US NIH, this requirement is known as the “Sex as a Biological Variable” or SABV policy, which went into effect in 2016. While laudable in its goals, SABV implementation has been spotty, and there is evidence that the large increase in number of researchers addressing sex differences without solid training (Gompers et al. 2024), understanding of gender/sex complexity (Pape et al. 2024), or use of appropriate statistical methods (Garcia‐Sifuentes and Maney 2021) has led to a preponderance of false positives that is not advancing the field as intended (Maney and Rich‐Edwards 2023).
This combination of cultural, institutional, and healthcare incentives has led to an outsized investment in neuroimaging studies of gender/sex differences over recent decades, with the vast majority of this research focused on binary brain sex differences. Whatever the imaging method employed, such studies can be neatly packaged into a simple template that invariably includes: (1) an Introduction that begins by citing gender disparities in mental health, especially anxiety, depression, and autism as crucial rationale for the research; (2) a Results section that focuses on whatever statistically significant differences were found in brain anatomy, function, or connectivity between binary male and female groups, often with small effect sizes, without proper controls for head size and other confounds, and without mention of the broad areas of male–female similarity and overlap; and (3) a Discussion or Conclusion that promises such findings will lead to advances in “personalized” or “precision” medicine to treat said disorders (e.g., Ferretti et al. 2018; Ritchie et al. 2018; Ryali et al. 2024; Wiersch et al. 2023).
This formula for binary sex comparison has been so widely exploited for funding and publications that purveyors can honestly claim that “thousands of studies” have uncovered sex differences in the human brain. However, closer inspection of the actual data across the largest and most high‐profile such studies reveals a string of small effect sizes, poor replication, and abandoned claims that add up to a cacophony of findings, rather than a clear, binary distinction between the brain organization of men and women, despite the 40 years of expensive effort (Eliot et al. 2021; Eliot 2024). The next two sections take an historical look at some of the higher profile such claims pursued over this period.
4. Claims of Brain Sex Difference in the Modern Era: Lateralization and Structural Volumes
Even before MRI took off as the preferred method, a flurry of post‐mortem studies in the 1980s claimed to discover one important sex difference in the brain: a larger corpus callosum in women. The original study was published in Science; although based on just 14 post‐mortem brains, it reported greater cross‐sectional width of the splenium of the five female donors, compared to the nine males, but no difference in the corpus callosum as a whole (de Lacoste‐Utamsing and Holloway 1982). Dozens more studies and several meta‐analyses followed, based on both autopsy and MRI measures, when the splenium finding did not replicate but it became apparent that the total volume or cross‐sectional area of the corpus callosum is actually larger in men—like all regional brain structures, since men's brains average about 11% greater volume than women's. This is a key confound that bedevils all male–female brain comparisons (see next section). Thus, it was found that once brain size is controlled, either by covarying with individuals' total intracranial volume (TIV) or by matching male and female subjects for total brain volume (Luders et al. 2014), the corpus callosum sex difference mostly disappears. Among the six largest MRI studies analyzed in Eliot et al. (2021), only one detected a small, significant sex difference, larger in females, with binary sex accounting for just 1% of the total variance in corpus callosum volume (Potvin et al. 2016, 2018).
Despite the weakness of the evidence, the claim that women have a relatively larger corpus callosum than men is often stated to be functionally important (e.g., Ingalhalikar et al. 2014). As the predominant route for interhemispheric traffic, a larger corpus callosum is said to permit less lateralized (more symmetrical) brain activity in women. This line of research also dates to the early 1980s and includes not only functional MRI studies but also electroencephalography (EEG), positron emission tomography (PET), and especially behavioral studies employing competing stimuli delivered to both sides of the body simultaneously (e.g., dichotic listening). Overall, the evidence across these approaches supports an extremely small difference in lateralization, greater in men, with binary sex accounting for just 0.1%–1.0% of total population variance (Boles 2005; Sommer et al. 2008; Voyer 2011). This conclusion has not been altered by the advent of very large MRI studies, including one with over 40 000 participants that for once reported on sex similarity, as opposed to difference: namely, a 98% correlation between men and women in the degree of asymmetry of individual brain regions (Williams et al. 2022). Moreover, the sexes are similar in showing about 1% larger volume of the right, compared to left hemispheres (Carne et al. 2006). For the handful of structures in which sex differences have been reported in the degree of lateralization, they often do not agree across large structural MRI studies (Kong et al. 2018; Guadalupe et al. 2017; Williams et al. 2022). Resting state functional MRI also finds overall similarity in the patterns of lateralized network organization between males and females (Nielsen et al. 2013). The very small and somewhat unreliable sex differences in brain lateralization explain why efforts to link hemispheric dominance to gender differences in cognitive performance have proven unsuccessful, according to a systematic review of some 40 years of research on this topic (Hirnstein et al. 2019).
After the corpus callosum, the most prominent claims about regional brain sex difference have focused on two limbic structures: the amygdala and hippocampus. In a highly influential review that is still widely cited, Cahill (2006) argued that “the sexually dimorphic nature” of these structures is crucial to understanding gender disparities in depression, PTSD and emotional memory. As evidence of sexual dimorphism, Cahill (2006) referenced one very small, early study (Goldstein et al. 2001) that was neither representative of the literature at that time nor held up to later replication in much larger studies (Eliot et al. 2021). Subsequent meta‐analyses found the hippocampus to be a non‐significantly 0.6% larger in women (Tan et al. 2016) and the amygdala, only 1.3% larger in men (Marwha et al. 2017) after accounting for individuals' total brain volume. The effect sizes were similarly negligible in the largest, most recent UK Biobank study that found minimal sex difference in hippocampal volume and a slightly larger amygdala in the left, but not right side of male brains (Williams et al. 2021). Nonetheless, claims about “sexual dimorphism” of the amygdala and hippocampus are still invoked as an explanation for gender disparities in mental health across both scientific (e.g., López‐Ojeda and Hurley 2021) and popular writing (e.g., Murray 2020).
Moving on to the neocortex, many dozens of studies have now examined sex differences in cortical measures, including the volume, surface area, and thickness of specific gyri. Here again, findings have been starkly unreliable: sex differences in specific gyri are small (Cohen's d < 0.20) and quite variable across the largest recent studies (reviewed in Eliot 2024). Some of this irreproducibility is likely attributable to methodological differences between studies, including scanner hardware, image pre‐processing, cortical segmentation, and global brain size correction methods. However, much of the data jitter likely also reflects real population differences: minute sex differences (Fjell et al. 2009) that are only detectable due to very large sample sizes, each with their own genetic and environmental idiosyncrasies that lead to a lack of concordance across populations (Yang et al. 2020).
Collectively, the massive literature on structural differences between male and female brains does not provide evidence for a species‐wide “sexual dimorphism”—literally, “two shapes”—such as ovaries versus testes. Rather, it indicates that the human brain is “sexually monomorphic,” similar to other non‐reproductive organs like the heart, liver, lungs, and kidneys. Each of these is some 15%–20% larger in men than in women (de la Grandmaison et al. 2001), but, like the brain, function similarly in both sexes, as evidenced by the fact that they can be transplanted between them with great success.
5. Brain Size, Sex “Prediction,” and the False Binarization of Brain Organization
Not to be deterred by unreliable findings on single brain structures, the search for binary brain sex difference took a new tack about a decade ago with the emergence of large‐scale multivariate analyses of neuroimaging data using ML tools. Whereas univariate approaches did not succeed in setting clear boundaries between the “male brain” and “female brain,” many research groups turned to ML tools to attempt to wrestle the hundreds of subtle and spatially complex morphological differences in any given MRI study into a classification system for distinguishing male and female brains. In a typical study, the algorithm is trained on the brain images of many hundreds of participants from a larger sample of up to thousands of individuals, half men and half women, and learns to find features that discriminate the “male” or “female” sex assigned to each scan. The trained algorithm is then tested on the remaining smaller subset and results are quantified as “sex prediction accuracy”—how precisely the model performs at identifying the known sex of each individual in the test set. Accuracies greater than 85% are common and often heralded as evidence for categorically different brains, or “dimorphism” (Feis et al. 2013; Anderson et al. 2019; Brennan et al. 2021), or promised to lead to “sex‐specific biomarkers” for mental disorders (Ryali et al. 2024). But keep in mind that chance prediction is 50% in such studies, and reported accuracies over 90% still mean that hundreds of males and females were mis‐identified in a large sample. This is not exactly the kind of “precision” that is envisioned for medical decision‐making, nor is the ability to predict an individual's sex from their brain scan of obvious utility for understanding or treating brain disorders. (There are much easier ways to discern a patient's sex.)
A further problem is that these classifications invariably perform poorly when the algorithms are applied to different cohorts, outside those used to train the model. Such cross‐validation is essential to proving species‐wide difference: that the structural or functional features used to discriminate male versus female brains in one human cohort are equally valid in another, as would be predicted if sexually distinct types of brains were selected through human evolution. However, the handful of studies that have tested their ML model against out‐of‐sample populations have seen their accuracy drop substantially (Joel et al. 2018; Ebel et al. 2023; Ryali et al. 2024), to as low as 57% in one study that compared several methods of controlling for brain size (Sanchis‐Segura et al. 2020). Then again, this imprecision is not surprising given that the specific brain foci identified as most important for sex prediction vary enormously across different studies, cohorts, and ML tools (Eliot et al. 2021; Lockhart 2023).
Once again, it is brain size that most confounds brain sex prediction studies, since this one, highly reliable sex difference influences most features of brain organization detectable by structural and functional MRI. According to the largest, UK Biobank study (Williams et al. 2021), men's brains average 124 mm3 or 11% larger than women's, not far off the “missing 5 ounces” (141 g) that stirred the fantasies of Victorian scientists, but a notably smaller sex difference than other non‐reproductive organs (de la Grandmaison et al. 2001). Among the sex prediction studies that have controlled for this difference in total brain volume, most find a reduction in accuracy down to the 60%–70% range (Chekroud et al. 2016; Sanchis‐Segura et al. 2020, 2022; More et al. 2021; Wiersch et al. 2023), even when the multivariate data are based on rsFC (using resting‐state fMRI [rsfMRI]) as opposed to just structural measures (Zhang et al. 2018). Indeed, the influence of brain size, most commonly quantified as TIV, is so strong that it alone can produce 84% accurate sex discrimination (Sanchis‐Segura et al. 2020).
There is no universal agreement about how to best control for TIV. Most brain features do not scale linearly, and even when nonlinear methods are used, there is clearly “feature leakage” or incomplete removal of size effects after most normalization procedures, biasing sex classification toward higher accuracy (More et al. 2021; Ebel et al. 2023; Wiersch et al. 2023). To overcome this problem, some ML studies have used training samples of men and women that are matched for TIV. Such samples have produced models that predict sex with over 90% accuracy within a similar cohort, but this drops to 80%–85% when tested in a non‐TIV matched sample (Ebel et al. 2023; Wiersch et al. 2023). Compared to more traditional methods of sex classification (e.g., chromosomes or genitalia), this is some two orders of magnitude less accurate, again showing that the massive multivariate brain features the models are detecting are not up to the task of “precision” medical diagnosis and treatment.
The reason brain size is so intrusive to sex classification is because it influences many features beyond structural volumes. Larger brains obviously have larger volumes of each compartment—white matter, gray matter, ventricles, and each cortical and subcortical zone—explaining the need to control for brain size in the studies described in the previous section. However, many measures do not scale linearly with brain size, such that linear covariance methods will not fully eliminate such size influences. This is especially true for the ratio of white: gray matter, which grows disproportionately with brain size. Overall, bigger brains require more or thicker myelinated axons to send information across longer distances, just as bigger cities require wider freeways to keep traffic flowing to faraway suburbs. On average, men's brains exhibit a 6% higher ratio of white‐to‐gray matter than women's brains (Leonard et al. 2008; Pintzka et al. 2015; Ritchie et al. 2018), as well as higher overall functional anisotropy (FA) and lower mean diffusivity (MD) as measured by DTI—all differences that have been shown to result from brain size, not sex per se (Takao et al. 2014; Eikenes et al. 2023). This means that women with bigger heads have higher FA and WM:GM ratio than women with smaller heads, and likewise for men of different sizes.
Another architectural feature that depends on brain size is the ratio of intra‐ to inter‐hemispheric connectivity, that is, the density of white matter pathways within each hemisphere versus their density within the corpus callosum that connects the two hemispheres. A high‐profile study of adolescents (Ingalhalikar et al. 2014) using DTI concluded that “male brains are optimized for intrahemispheric and female brains for interhemispheric communication” based on significant sex differences in a subset of each type of pathway. However, there were major limitations to this study. Not only were the differences small and the participants not matched for the 1–2 years difference in pubertal stage between boys and girls, the study included no control for brain size, which other research has shown to fully account for the apparent sex difference (Hänggi et al. 2014). That is, bigger brains have proportionally less white matter connecting the two hemispheres, despite having more white matter overall, another architectural constraint that has to do with the lower efficiency of callosal transmission with increasing brain size (Ringo et al. 1994). So while this architecture does indeed produce an average sex difference in the intra‐to‐interhemispheric connectivity ratio, it does not support the highly speculative conclusion of Ingalhalikar et al. that “male brains are structured to facilitate connectivity between perception and coordinated action, whereas female brains are designed to facilitate communication between analytical and intuitive processing modes.” A more accurate extrapolation would be that “larger brains are designed for action and smaller brains are designed for intuition,” an absurd evolutionary proposition that illustrates the problem of cherry‐picking when theorizing about the behavior relevance of select brain findings.
Instead, this research tells us that what looks to be a male–female brain difference often turns out to be a function of size, not sex, and therefore not informative for gender differences in behavior and mental health.
6. Attempts to Add Gender to the Mix: Brain Imaging Using Non‐Binary Independent Variables
As this brief history reveals, little has changed in the search for and framing of human brain structural sex differences over the modern era. The simple design of sorting participants into M and F groups (mostly by self‐identification on intake forms) remains irresistible to imaging researchers seeking to find some neural basis for well‐known gender behavior and mental health differences. But while the sample sizes have grown impressively large, and imaging analysis has grown increasingly sophisticated, the findings of this widespread research have not achieved their aim of uncovering reproducible brain sex differences that can account for gender behavioral differences. One possible reason is that such binary, sex‐based sorting is far too crude compared to the wide variation of gendered behavior within and across sexes. For example, men are more aggressive than women across most ages and cultures (Archer 2004), but the difference is more closely related to gender role orientation than to binary sex categorization, requiring a more nuanced approach to its neural analysis (Li et al. 2024).
In other words, we have these two convenient biological categories, but they do not adequately align with the mosaic of gender identities, expression, and social roles that vary both within and between individuals (Rippon et al. 2014). When neuroimaging does take account of individual differences within each sex, as opposed to just average group‐level differences, every individual shows some mosaic of “male‐like” and “female‐like” structural volumes, regardless of binary sex assignment (Joel et al. 2015). Thus, binary sex is a poor way of sorting participants in neuroimaging research when it is really the mosaic of gendered behavior that we seek to understand.
To address this problem, a few studies dating back to 2008 have tried using non‐binary measures of gender as an independent variable for analyzing brain organization. Such research requires administering one of several validated gender inventories (questionnaires where participants are asked to self‐rate various aspects of gender typicality) in concert with brain imaging to determine whether gender attributes, alone or in addition to binary sex, are associated with specific brain measures. A systematic literature review uncovered just eight small studies taking this approach prior to 2022, with a scattering of findings suggesting that gender attributes do subtly moderate measures of brain structure or rsfMRI activity, particularly in the frontal lobe (Rauch and Eliot 2022). In addition, this review identified three larger studies taking an opposite approach: all began with brain measures, constructing a non‐binary spectrum based on dozens‐to‐hundreds of specific differences, and then correlated these brain spectra to one particular dimension of masculine‐feminine psychological difference, internalizing versus externalizing behaviors. This dimension is of interest because internalizing behaviors are associated with anxiety and depression, which are more often diagnosed in women, whereas externalizing behaviors are associated with antisocial and substance use disorders that are more commonly diagnosed in men (Kramer et al. 2008). Despite the general similarity of approach, cohorts in the three studies differed substantially in age and in the measures used to construct brain sex/gender spectra—from resting state functional MRI (Zhang et al. 2021) to structural and diffusion MRI measures (Phillips et al. 2019), to a combination of bodily and brain measures (Vosberg et al. 2021)—so the three studies cannot be compared for replication.
The most recent research takes a more global approach to testing the impact of gender on brain‐wide measures and has largely focused on preadolescents. Using rsfMRI to assesses rsFC patterns in the large ABCD (2022) cohort of 9‐ to 10‐year‐olds, Dhamala et al. (2024) found that different brain networks were associated with sex versus gender identity. In this study, ML algorithms could predict sex quite accurately (albeit without controlling for TIV), but gender prediction accuracy was considerably lower and notably, only worked when gender was assessed using a 12‐item parent report; the brain model could not discriminate gender based on a simpler survey of the preadolescents themselves that asked about “felt gender, gender expression and gender contentedness” (Potter et al. 2022). Although providing some support for the notion that gender is encoded differently from sex in neural networks, the weakness of the gender‐brain correlations, together with the lack of control for brain size (Qing and Gong 2016) leaves considerable ambiguity about the basis of gender‐based brain correlates. Indeed, a preliminary report using the same ABCD database and similar rsFC analysis was unable to predict individual youth's gender scores, measured as a composite of the same child‐ and parent‐completed surveys (Metoki et al. 2024). These authors concluded that “gender may be a more complex construct that is not as clearly reflected in functional connectivity or cortical thickness patterns.”
A similar conclusion is supported by Torgerson et al. (2024), who used the structural, as opposed to functional MRI data in the ABCD cohort (Casey et al. 2018) to compare the influences of sex versus “felt‐gender score” on 9‐ and 11‐year‐olds' brain metrics. The study found, first, that the effects of sex alone on brain structures were “negligible to small” after accounting for TIV, in agreement with adult studies described above. With regard to gender, Torgerson et al. (2024) were unable to identify any gray or white matter regions whose volume was predicted by youths' gender score, concluding that “gender diversity is not directly associated with neurostructural diversity.” Of note, Torgerson et al. (2024) did not utilize the parent assessment that proved more sensitive to gender variation in Dhamala et al. (2024), so it is possible that brain structural variance may show a relationship to youth gender expression based on this instrument.
In sum, the substitution of gender for sex as an independent variable in brain imaging studies is still very new but does not seem poised to add much insight into brain organization. One key limitation is the gender surveys themselves, since such research is highly dependent on the availability of modern, well‐validated, and easy to administer instruments for operationalizing gender. Among the brain imaging studies captured by Rauch and Eliot (2022), several used the Bem Sex Role Inventory (BSRI) or the Personal Attributes Questionnaire (PAQ), gender instruments developed in the 1970s that are based on outdated notions of masculinity and femininity (Nielsen et al. 2021) but are still widely used in health research (Horstmann et al. 2022). The ABCD project has generated its own, updated gender instruments, but the youth self‐report version produces a very narrow range of average measures, close to the maximum score for both males and females (Potter et al. 2022), perhaps reflecting the strong pressure for gender conformity in childhood and early adolescence. The longer parent version detects a wider range of youth gender expression and dysphoria, which may explain its better ability to predict some of the variance in brain connectivity, compared to no prediction for the youth version (Dhamala et al. 2024; Metoki et al. 2024; Torgerson et al. 2024). Nonetheless, it is clear that existing instruments are capturing just a piece of the complex construct of gender and may not be quantifying the elements that will be most relatable to neural circuitry or mental health disparities.
7. Beyond Gender or Sex as Essential Variables for Brain Health Research
As defined above, gender is both a social and a cultural variable that operates on many scales capable of impacting human brains: from individual identity and expression to the structure of labor markets, expectations about autonomy and caregiving, and experiences of violence and discrimination (Bolte et al. 2021; Miani et al. 2021). None of these influences is binary, nor do they map onto the dozen or so canonical brain networks (Gordon et al. 2017) that form the backbone of FC (e.g., default mode, frontoparietal, dorsal attention, language, salience, somatomotor, visual, auditory, etc.). Despite the wide‐ranging nature of gender, most of the instruments for operationalizing it focus on identity and conformity, and their construction necessarily entails a trade‐off between quantifying gender expression versus reifying binary stereotypes by implicitly labeling traits as masculine or feminine (Horstmann et al. 2022). One newer instrument that has explicitly avoided such bipolar spectra is the Stanford Gender‐related Variables for Health Research (GVHR; Nielsen et al. 2021). Constructed and validated from a factor analysis of 74 existing gender scales, the GVHR is focused on three domains (gender norms, gender traits and gender relations) and minimizes acquiescence bias by avoiding “agree‐disagree” scales and by querying participants about behaviors and experiences as opposed to self‐perceived attributes. However, this scale has not yet been used in brain imaging studies, nor is it suitable for child or adolescent cohorts.
In this context, it is important to mention another approach for assessing neural correlates of gender that has been widely used, but is increasingly recognized as problematic: neuroimaging focused on transgender participants. The rationale for such studies is that by comparing brain structure or connectivity between groups of cisgender and transgender individuals of the same birth‐assigned sex, researchers might uncover some neural signature of gender identity, unencumbered by sex. This approach emerged in 1995 with one very small, high‐profile post‐mortem study (Zhou et al. 1995), but mostly took off around 2009 when MRI began to be applied to the question. By, Smith et al. (2015) reviewed some 18 studies in this genre and, noting the lack of replication among them, concluded that “viewing gender as a binary or dichotomous category has to be abandoned.” Eight years later, the conclusion remained the same in a review by Levin et al. (2023), now including 49 neuroimaging studies. Not only has there been little replication across these dozens of studies, several are overtly contradictory and the entire enterprise suffers from implicitly pathologizing transgender identities. Levin et al. (2023) especially critique efforts to use ML for discriminating trans‐ from cisgender individuals' brains, research that assumes “biological features can serve as a simple proxy for social and cultural identities” in much the same what that ML has been recklessly applied to racial and ethnic categorizations (Zou and Schiebinger 2018). Like other critics of this research (Llaveria Caselles 2021; DuBois and Shattuck‐Heidorn 2021), Levin and colleagues recommend that any future studies include transgender community members as part of the research team, to curtail its stigmatizing aims and produce data of greater value to transgender people's health.
Whether studying cisgender, transgender, or ideally a broad sample of individuals representing the true diversity of gender experience, it seems clear that neither binary categories nor a linear spectrum will suffice to operationalize it. As a multidimensional construct, gender is increasingly recognized as a mosaic of traits, roles, behaviors, and attitudes that vary within individuals and across time and cannot be reduced to any single scale (Rippon et al. 2014). This recognition calls for an entirely different approach to the study of gender/sex brain health disparities. Rather than attempting to link brain measures to either gender or sex as global variables, this approach calls for a more targeted effort to link specific sex‐ or gender‐related behaviors or attributes to brain measures. For example, since men and women differ, on average, in susceptibility to clinical depression, research can focus on the neural markers of a depressed state and ask secondarily if such markers differ between men and women. Using this approach, a recent analysis of FC patterns identified six distinct biotypes in depressed patients, none of which were more common in women or men (Tozzi et al. 2024). This pathway for gender/sex analysis—from behavior to brain, rather than brain to behavior—makes it less likely that any particular neural difference will be cherry‐picked to explain a particular behavioral gender difference.
Another solution to the multidimensionality of both sex and gender is to cease using these as variables at all. Instead, researchers are increasingly recommending a shift to individual elements underlying sex and gender categories, which may be more informative for understanding specific brain measures (DiMarco et al. 2022; Wierenga et al. 2023). In lieu of sex, research can focus on particular chromosome configurations, gene expression patterns, hormone levels, or body size and composition (Pape et al. 2024) as independent variables. In lieu of gender, analysis could focus on specific dimensions of psychology (e.g., anxiety, emotionality, risk‐taking; Kajonius and Johnson 2018), social role (caregiver, supervisor, subordinate; Suo et al. 2012; Rilling et al. 2025), economic status (income, financial security; Roy and Chaudhuri 2008), life experience (education, occupation, leisure pursuits, physical activity; Halliday et al. 2019), or lifetime exposures (nutrition, environmental toxins, harassment, discrimination, violence; Bucher et al. 2023) that typically differ between women and men or boys and girls. Such findings would not only provide a deeper understanding of brain‐behavior relationships but may also produce more actionable evidence for addressing brain health in diverse populations.
Looking ahead, the search for neural correlates of gender behavioral differences can take its cues from recent shifts in addressing racial health disparities. Confronted by profound differences in prevalence and outcomes of many diseases between self‐identified Black and White populations (e.g., hypertension, cancer, maternal morbidity), researchers and clinicians no longer look automatically to genetic differences between Black and White populations, but are now likelier to explore the social determinants of health, including systemic racism (Phelan and Link 2015). Such change has been decades in the making and still faces substantial hurdles (Bird and Carlson 2024) but is widely endorsed by medical and scientific leaders (American Medical Association 2021; Pérez‐Stable and Webb Hooper 2023). Of particular concern is the way in which genetic population descriptors obscure local variance and reinforce myths of “pure” population distinctions, promoting “typological thinking” about groups (US National Academies of Sciences, Engineering, and Medicine 2022). Similar typological views about men and women, also known as “gender essentialism,” have long been fostered by research on brain sex differences (Fine 2014; O'Connor and Joffe 2014; Eliot et al. 2023) and demand a similar re‐tooling of our approach to gender behavioral health disparities.
8. Conclusion
A topic of ancient fascination, male–female brain difference has in recent decades been avidly pursued by neuroimaging researchers, often in the name of “precision medicine.” On the whole, studies of brain gender/sex difference have done more to falsely binarize our understanding of brain organization than to elucidate its relationship to behavior and mental health outcomes in women and men. Most of the research has focused on binary sex categories, cataloging small brain differences that have not reproduced well across diverse populations. Indeed, this large research project provides stronger evidence that the human brain is “sexually monomorphic” than “dimorphic.” Attempts to replace sex with simple gender measures or linear masculine–feminine spectra are still new in brain imaging but do not appear any more promising for mapping brain to behavior. Given the multidimensional nature of both sex and gender, true precision may require abandoning both global variables and focusing instead on specific biological and social factors that shape brain mechanisms underlying individuals' behavior and mental health.
Ethics Statement
The author has nothing to report.
Conflicts of Interest
The author declares no conflicts of interest.
Acknowledgments
This research was supported by a grant from the Fred B. Snite Foundation.
Funding: This research was supported by Fred B. Snite Foundation.
Data Availability Statement
Data sharing not applicable to this article as no datasets were generated or analysed during the current study.
References
- ABCD . 2022. “ABCD Study.” https://abcdstudy.org/about/.
- American Medical Association . 2021. Advancing Health Equity: A Guide to Language, Narrative and Concepts. AMA Center for Health Equality. https://www.ama‐assn.org/about/ama‐center‐health‐equity/advancing‐health‐equity‐guide‐language‐narrative‐and‐concepts‐0. [Google Scholar]
- Anderson, N. E. , Harenski K. A., Harenski C. L., et al. 2019. “Machine Learning of Brain Gray Matter Differentiates Sex in a Large Forensic Sample.” Human Brain Mapping 40: 1–11. 10.1002/hbm.24462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Archer, J. 2004. “Sex Differences in Aggression in Real‐World Settings: A Meta‐Analytic Review.” Review of General Psychology 8: 291–322. 10.1037/1089-2680.8.4.291. [DOI] [Google Scholar]
- Barkow, J. , Cosmides L., and Tooby J.. 1992. The Adapted Mind: Evolutionary Psychology and the Generation of Culture. Oxford University Press. [Google Scholar]
- Beery, A. K. , and Zucker I.. 2011. “Sex Bias in Neuroscience and Biomedical Research.” Neuroscience & Biobehavioral Reviews 35: 565–572. 10.1016/j.neubiorev.2010.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswal, B. B. , Mennes M., Zuo X. N., et al. 2010. “Toward Discovery Science of Human Brain Function.” Proceedings of the National Academy of Sciences of the United States of America 107: 4734–4739. 10.1073/pnas.0911855107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bird, K. A. , and Carlson J.. 2024. “Typological Thinking in Human Genomics Research Contributes to the Production and Prominence of Scientific Racism.” Frontiers in Genetics 15: 1345631. 10.3389/fgene.2024.1345631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boles, D. B. 2005. “A Large‐Sample Study of Sex Differences in Functional Cerebral Lateralization.” Journal of Clinical and Experimental Neuropsychology 27: 759–768. 10.1081/13803390590954263. [DOI] [PubMed] [Google Scholar]
- Bolte, G. , Jacke K., Groth K., et al. 2021. “Integrating Sex/Gender Into Environmental Health Research: Development of a Conceptual Framework.” International Journal of Environmental Research and Public Health 18: 12118. 10.3390/ijerph182212118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennan, D. , Wu T., and Fan J.. 2021. “Morphometrical Brain Markers of Sex Difference.” Cerebral Cortex 31: 3641–3649. 10.1093/cercor/bhab037. [DOI] [PubMed] [Google Scholar]
- Briggs, F. B. , and Hill E.. 2020. “Estimating the Prevalence of Multiple Sclerosis Using 56.6 Million Electronic Health Records From the United States.” Multiple Sclerosis Journal 26: 1948–1952. 10.1177/135245851986468. [DOI] [PubMed] [Google Scholar]
- Bucher, M. L. , Anderson F. L., Lai Y., Dicent J., Miller G. W., and Zota A. R.. 2023. “Exposomics as a Tool to Investigate Differences in Health and Disease by Sex and Gender.” Exposome 3: osad003. 10.1093/exposome/osad003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckley, R. F. , Waller M., Masters C. L., and Dobson A.. 2019. “To What Extent Does Age at Death Account for Sex Differences in Rates of Mortality From Alzheimer Disease?” American Journal of Epidemiology 188: 1213–1223. 10.1093/aje/kwz048. [DOI] [PubMed] [Google Scholar]
- Cahill, L. 2006. “Why Sex Matters for Neuroscience.” Nature Reviews Neuroscience 7: 477–484. 10.1038/nrn1909. [DOI] [PubMed] [Google Scholar]
- Cahill, L. 2014. “Equal≠ the Same: Sex Differences in the Human Brain.” Cerebrum: The Dana Forum on Brain Science 2014: 5. [PMC free article] [PubMed] [Google Scholar]
- Carne, R. P. , Vogrin S., Litewka L., and Cook M. J.. 2006. “Cerebral Cortex: An MRI‐Based Study of Volume and Variance With Age and Sex.” Journal of Clinical Neuroscience 13: 60–72. 10.1016/j.jocn.2005.02.013. [DOI] [PubMed] [Google Scholar]
- Casey, B. J. , Cannonier T., Conley M. I., et al. 2018. “The Adolescent Brain Cognitive Development (ABCD) Study: Imaging Acquisition Across 21 Sites.” Developmental Cognitive Neuroscience 32: 43–54. 10.1016/j.dcn.2018.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chekroud, A. M. , Ward E. J., Rosenberg M. D., and Holmes A. J.. 2016. “Patterns in the Human Brain Mosaic Discriminate Males From Females.” Proceedings of the National Academy of Sciences of the United States of America 113: E1968. 10.1073/pnas.1523888113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Grandmaison, G. L. , Clairand I., and Durigon M.. 2001. “Organ Weight in 684 Adult Autopsies: New Tables for a Caucasoid Population.” Forensic Science International 119: 149–154. 10.1016/S0379-0738(00)00401-1. [DOI] [PubMed] [Google Scholar]
- de Lacoste‐Utamsing, C. , and Holloway R. L.. 1982. “Sexual Dimorphism in the Human Corpus Callosum.” Science 216: 1431–1432. 10.1126/science.7089533. [DOI] [PubMed] [Google Scholar]
- DeCasien, A. R. , Guma E., Liu S., and Raznahan A.. 2022. “Sex Differences in the Human Brain: A Roadmap for More Careful Analysis and Interpretation of a Biological Reality.” Biology of Sex Differences 13: 43. 10.1186/s13293-022-00448-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhamala, E. , Bassett D. S., Yeo B. T., and Holmes A. J.. 2024. “Functional Brain Networks Are Associated With Both Sex and Gender in Children.” Science Advances 10: eadn4202. 10.1126/sciadv.adn4202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiMarco, M. , Zhao H., Boulicault M., and Richardson S. S.. 2022. “Why “Sex as a Biological Variable” Conflicts With Precision Medicine Initiatives.” Cell Reports Medicine 3: 100550. 10.1016/j.xcrm.2022.100550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DuBois, L. Z. , and Shattuck‐Heidorn H.. 2021. “Challenging the Binary: Gender/Sex and the Bio‐Logics of Normalcy.” American Journal of Human Biology 33: e23623. 10.1002/ajhb.23623. [DOI] [PubMed] [Google Scholar]
- Ebel, M. , Domin M., Neumann N., Schmidt C. O., Lotze M., and Stanke M.. 2023. “Classifying Sex With Volume‐Matched Brain MRI.” Neuroimage: Reports 3: 100181. 10.1016/j.ynirp.2023.100181. [DOI] [Google Scholar]
- Eikenes, L. , Visser E., Vangberg T., and Håberg A. K.. 2023. “Both Brain Size and Biological Sex Contribute to Variation in White Matter Microstructure in Middle‐Aged Healthy Adults.” Human Brain Mapping 44: 691–709. 10.1002/hbm.26093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eliot, L. 2011. “The Trouble With Sex Differences.” Neuron 72: 895–898. 10.1016/j.neuron.2011.12.001. [DOI] [PubMed] [Google Scholar]
- Eliot, L. 2024. “Remembering the Null Hypothesis When Searching for Brain Sex Differences.” Biology of Sex Differences 15: 14. 10.1186/s13293-024-00585-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eliot, L. , Ahmed A., Khan H., and Patel J.. 2021. “Dump the ‘Dimorphism’: Comprehensive Synthesis of Human Brain Studies Reveals Few Male‐Female Differences Beyond Size.” Neuroscience & Biobehavioral Reviews 125: 667–697. 10.1016/j.neubiorev.2021.02.026. [DOI] [PubMed] [Google Scholar]
- Eliot, L. , Beery A. K., Jacobs E. G., LeBlanc H. F., Maney D. L., and McCarthy M. M.. 2023. “Why and How to Account for Sex and Gender in Brain and Behavioral Research.” Journal of Neuroscience 43: 6344–6356. 10.1523/JNEUROSCI.0020-23.2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feis, D. , Brodersen K. H., von Cramon D. Y., Luders E., and Tittgemeyer M.. 2013. “Decoding Gender Dimorphism of the Human Brain Using Multimodal Anatomical and Diffusion MRI Data.” NeuroImage 70: 250–257. 10.1016/j.neuroimage.2012.12.068. [DOI] [PubMed] [Google Scholar]
- Ferretti, M. T. , Iulita M. F., Cavedo E., et al. 2018. “Sex Differences in Alzheimer Disease—The Gateway to Precision Medicine.” Nature Reviews Neurology 14: 457–469. 10.1038/s41582-018-0032-9. [DOI] [PubMed] [Google Scholar]
- Fine, C. 2010. “From Scanner to Sound Bite: Issues in Interpreting and Reporting Sex Differences in the Brain.” Current Directions in Psychological Science 19: 280–283. 10.1177/0963721410383248. [DOI] [Google Scholar]
- Fine, C. 2014. “His Brain, Her Brain?” Science 346: 915–916. 10.1126/science.1262061. [DOI] [PubMed] [Google Scholar]
- Fjell, A. M. , Westlye L. T., Amlien I., et al. 2009. “Minute Effects of Sex on the Aging Brain: A Multisample Magnetic Resonance Imaging Study of Healthy Aging and Alzheimer’s Disease.” Journal of Neuroscience 29: 8774–8783. 10.1523/jneurosci.0115-09.2009.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuentes, A. 2021. “The Descent of Man, 150 Years on.” Science 372: 769. 10.1126/science.abj46. [DOI] [PubMed] [Google Scholar]
- Garcia‐Sifuentes, Y. , and Maney D. L.. 2021. “Reporting and Misreporting of Sex Differences in the Biological Sciences.” eLife 10: e70817. 10.7554/eLife.70817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glick, P. , and Fiske S. T.. 1997. “Hostile and Benevolent Sexism: Measuring Ambivalent Sexist Attitudes Toward Women.” Psychology of Women Quarterly 21: 119–135. 10.1111/j.1471-6402.1997.tb00104. [DOI] [Google Scholar]
- Goldstein, J. M. , Seidman L. J., Horton N. J., et al. 2001. “Normal Sexual Dimorphism of the Adult Human Brain Assessed by In Vivo Magnetic Resonance Imaging.” Cerebral Cortex 11: 490–497. 10.1093/cercor/11.6.490. [DOI] [PubMed] [Google Scholar]
- Gompers, A. , Olivier M. T., and Maney D. L.. 2024. “Training in the Implementation of Sex and Gender Research Policies: An Evaluation of Publicly Available Online Courses.” Biology of Sex Differences 15: 32. 10.1186/s13293-024-00610-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon, E. M. , Laumann T. O., Gilmore A. W., et al. 2017. “Precision Functional Mapping of Individual Human Brains.” Neuron 95: 791–807. 10.1016/j.neuron.2017.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould, S. J. 1980. The Panda's Thumb: More Reflections in Natural H History, 153. WW Norton. [Google Scholar]
- Grant, B. F. , Dawson D. A., Stinson F. S., Chou S. P., Dufour M. C., and Pickering R. P.. 2004. “The 12‐Month Prevalence and Trends in DSM‐IV Alcohol Abuse and Dependence: United States, 1991–1992 and 2001–2002.” Drug and Alcohol Dependence 74: 223–234. 10.1016/j.drugalcdep.2004.02.004. [DOI] [PubMed] [Google Scholar]
- Gray, J. 1992. Men Are From Mars, Women Are From Venus: The Definitive Guide to Relationships. Harper Collins. [Google Scholar]
- Guadalupe, T. , Mathias S. R., Vanerp T. G., et al. 2017. “Human Subcortical Brain Asymmetries in 15,847 People Worldwide Reveal Effects of Age and Sex.” Brain Imaging and Behavior 11, no. 5: 1497–1514. 10.1007/s11682-016-9629-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gur, R. C. , and Gur R. E.. 2017. “Complementarity of Sex Differences in Brain and Behavior: From Laterality to Multimodal Neuroimaging.” Journal of Neuroscience Research 95: 189–199. 10.1002/jnr.23830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halliday, A. J. , Kern M. L., and Turnbull D. A.. 2019. “Can Physical Activity Help Explain the Gender Gap in Adolescent Mental Health? A Cross‐Sectional Exploration.” Mental Health and Physical Activity 16: 8–18. 10.1016/j.mhpa.2019.02.003. [DOI] [Google Scholar]
- Halpern, D. F. 2010. “How Neuromythologies Support Sex Role Stereotypes.” Science 339: 1320–1321. 10.1126/science.1198057. [DOI] [Google Scholar]
- Hänggi, J. , Fövenyi L., Liem F., Meyer M., and Jäncke L.. 2014. “The Hypothesis of Neuronal Interconnectivity as a Function of Brain Size—A General Organization Principle of the Human Connectome.” Frontiers in Human Neuroscience 8: 915. 10.3389/fnhum.2014.00915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirnstein, M. , Hugdahl K., and Hausmann M.. 2019. “Cognitive Sex Differences and Hemispheric Asymmetry: A Critical Review of 40 Years of Research.” Laterality: Asymmetries of Body, Brain and Cognition 24: 204–252. 10.1080/1357650X.2018.1497044. [DOI] [PubMed] [Google Scholar]
- Horstmann, S. , Schmechel C., Palm K., Oertelt‐Prigione S., and Bolte G.. 2022. “The Operationalisation of Sex and Gender in Quantitative Health–Related Research: A Scoping Review.” International Journal of Environmental Research and Public Health 19: 7493. 10.3390/ijerph19127493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson, J. I. , Hiripi E., Pope H. G. Jr., and Kessler R. C.. 2007. “The Prevalence and Correlates of Eating Disorders in the National Comorbidity Survey Replication.” Biological Psychiatry 61: 348–358. 10.1016/j.biopsych.2006.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyde, J. S. , Bigler R. S., Joel D., Tate C. C., and van Anders S. M.. 2019. “The Future of Sex and Gender in Psychology: Five Challenges to the Gender Binary.” American Psychologist 74: 171–193. 10.1037/amp0000307. [DOI] [PubMed] [Google Scholar]
- Ingalhalikar, M. , Smith A., Parker D., et al. 2014. “Sex Differences in the Structural Connectome of the Human Brain.” Proceedings of the National Academy of Sciences of the United States of America 111: 823–828. 10.1073/pnas.1316909110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joel, D. , Berman Z., Tavor I., et al. 2015. “Sex Beyond the Genitalia: The Human Brain Mosaic.” Proceedings of the National Academy of Sciences of the United States of America 112: 15468–15473. 10.1073/pnas.1509654112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joel, D. , Persico A., Salhov M., et al. 2018. “Analysis of Human Brain Structure Reveals That the Brain ‘Types’ Typical of Males Are Also Typical of Females, and Vice Versa.” Frontiers in Human Neuroscience 12: 399. 10.3389/fnhum.2018.00399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kajonius, P. J. , and Johnson J.. 2018. “Sex Differences in 30 Facets of the Five Factor Model of Personality in the Large Public (N = 320,128).” Personality and Individual Differences 129: 126–130. 10.1016/j.paid.2018.03.026. [DOI] [Google Scholar]
- Kong, X. Z. , Mathias S. R., Guadalupe T., et al. 2018. “Mapping Cortical Brain Asymmetry in 17,141 Healthy Individuals Worldwide via the ENIGMA Consortium.” Proceedings of the National Academy of Sciences of the United States of America 115, no. 22: E5154–E5163. 10.1073/pnas.1718418115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer, D. M. , Krueger F. R., and Hicks M. B.. 2008. “The Role of Internalizing and Externalizing Liability Factors in Accounting for Gender Differences in the Prevalence of Common Psychopathological Syndromes.” Psychological Medicine 38: 51–61. 10.1017/S0033291707001572. [DOI] [PubMed] [Google Scholar]
- Lauterbur, P. 1973. “Image Formation by Induced Local Interactions: Examples Employing Nuclear Magnetic Resonance.” Nature 242: 190–191. 10.1038/242190a0. [DOI] [PubMed] [Google Scholar]
- Leonard, C. M. , Towler S., Welcome S., et al. 2008. “Size Matters: Cerebral Volume Influences Sex Differences in Neuroanatomy.” Cerebral Cortex 18: 2920–2931. 10.1093/cercor/bhn052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin, R. N. , Erickson‐Schroth L., Mak K., and Edmiston E. K.. 2023. “Biological Studies of Transgender Identity: A Critical Review.” Journal of Gay & Lesbian Mental Health 27: 254–283. 10.1080/19359705.2022.2127042. [DOI] [Google Scholar]
- Li, Z. , Liu Y., Liu W., and Chen H.. 2024. “Is Being Male a Marker of Aggression? Evidence for the Decoupling of Sex and Gender Role Orientation.” Brain Sciences 14: 1176. 10.3390/brainsci14121176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llaveria Caselles, E. 2021. “Epistemic Injustice in Brain Studies of (Trans) Gender Identity.” Frontiers in Sociology 6: 63. 10.3389/fsoc.2021.608328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lockhart, J. W. 2023. “Because the Machine Can Discriminate: How Machine Learning Serves and Transforms Biological Explanations of Human Difference.” Big Data & Society 10: 20539517231155060. 10.1177/20539517231155060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loomes, R. , Hull L., and Mandy W. P.. 2017. “What Is the Male‐To‐Female Ratio in Autism Spectrum Disorder? A Systematic Review and Meta‐Analysis.” Journal of the American Academy of Child & Adolescent Psychiatry 56: 466–474. 10.1016/j.jaac.2017.03.013. [DOI] [PubMed] [Google Scholar]
- López‐Ojeda, W. , and Hurley R. A.. 2021. “Sexual Dimorphism in Brain Development: Influence on Affective Disorders.” Journal of Neuropsychiatry and Clinical Neurosciences 33: A4‐85. 10.1176/appi.neuropsych.20100269. [DOI] [PubMed] [Google Scholar]
- Luders, E. , Toga A. W., and Thompson P. M.. 2014. “Why Size Matters: Differences in Brain Volume Account for Apparent Sex Differences in Callosal Anatomy: The Sexual Dimorphism of the Corpus Callosum.” NeuroImage 84: 820–824. 10.1016/j.neuroimage.2013.09.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maney, D. L. 2015. “Just Like a Circus: The Public Consumption of Sex Differences.” Current Topics in Behavioral Neurosciences 19: 279–296. 10.1007/7854_2014_339. [DOI] [PubMed] [Google Scholar]
- Maney, D. L. , and Rich‐Edwards J. W.. 2023. “Sex‐Inclusive Biomedicine: Are New Policies Increasing Rigor and Reproducibility?” Women's Health Issues 33: 461–464. 10.1016/j.whi.2023.03.004. [DOI] [PubMed] [Google Scholar]
- Marwha, D. , Halari M., and Eliot L.. 2017. “Meta‐Analysis Reveals a Lack of Sexual Dimorphism in Human Amygdala Volume.” NeuroImage 147: 282–294. 10.1016/j.neuroimage.2016.12.021. [DOI] [PubMed] [Google Scholar]
- McLean, C. P. , Asnaani A., Litz B. T., and Hofmann S. G.. 2011. “Gender Differences in Anxiety Disorders: Prevalence, Course of Illness, Comorbidity and Burden of Illness.” Journal of Psychiatric Research 45: 1027–1035. 10.1016/j.jpsychires.2011.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metoki, A. , Chauvin R. J., Gordon E. M., et al. 2024. “Brain Functional Connectivity, but Not Neuroanatomy, Captures the Interrelationship Between Sex and Gender in Preadolescents.” bioRxiv: 2024.10.31.621379. 10.1101/2024.10.31.621379. [DOI] [Google Scholar]
- Miani, C. , Wandschneider L., Niemann J., Batram‐Zantvoort S., and Razum O.. 2021. “Measurement of Gender as a Social Determinant of Health in Epidemiology: A Scoping Review.” PLoS One 16: e0259223. 10.1371/journal.pone.0259223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller, V. M. , Rocca W. A., and Faubion S. S.. 2015. “Sex Differences Research, Precision Medicine, and the Future of Women's Health.” Journal of Women's Health 24: 969–971. 10.1089/jwh.2015.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- More, S. , Eickhoff S. B., Caspers J., and Patil K. R.. 2021. “Confound Removal and Normalization in Practice: A Neuroimaging Based Sex Prediction Case Study.” In Machine Learning and Knowledge Discovery in Databases. Applied Data Science and Demo Track, 3–18. Springer International. 10.1007/978-3-030-67670-4_1. [DOI] [Google Scholar]
- Murray, C. A. 2020. Human Diversity: The Biology of Gender, Race, and Class. Twelve. [Google Scholar]
- Nielsen, J. A. , Zielinski B. A., Ferguson M. A., Lainhart J. E., and Anderson J. S.. 2013. “An Evaluation of the Left‐Brain vs. Right‐Brain Hypothesis With Resting State Functional Connectivity Magnetic Resonance Imaging.” PLoS One 8: 1–11. 10.1371/journal.pone.0071275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen, M. W. , Stefanick M. L., Peragine D., et al. 2021. “Gender‐Related Variables for Health Research.” Biology of Sex Differences 12: 1–16. 10.1186/s13293-021-00366-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connor, C. , and Joffe H.. 2014. “Gender on the Brain: A Case Study of Science Communication in the New Media Environment.” PLoS One 9: e110830. 10.1371/journal.pone.0110830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pape, M. , Miyagi M., Ritz S. A., Boulicault M., Richardson S. S., and Maney D. L.. 2024. “Sex Contextualism in Laboratory Research: Enhancing Rigor and Precision in the Study of Sex‐Related Variables.” Cell 187: 1316–1326. 10.1016/j.cell.2024.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez‐Stable, E. J. , and Webb Hooper M.. 2023. “The Pillars of Health Disparities Science—Race, Ethnicity, and Socioeconomic Status.” Journal of the American Medical Association Health Forum 4: e234463. 10.1001/jamahealthforum.2023.4463. [DOI] [PubMed] [Google Scholar]
- Phelan, J. C. , and Link B. G.. 2015. “Is Racism a Fundamental Cause of Inequalities in Health?” Annual Review of Sociology 41: 311–330. 10.1146/annurev-soc-073014-112305. [DOI] [Google Scholar]
- Phillips, O. R. , Onopa A. K., Hsu V., et al. 2019. “Beyond a Binary Classification of Sex: An Examination of Brain Sex Differentiation, Psychopathology, and Genotype.” Journal of the American Academy of Child & Adolescent Psychiatry 58: 787–798. 10.1016/j.jaac.2018.09.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pintzka, C. W. , Hansen T. I., Evensmoen H. R., and Håberg A. K.. 2015. “Marked Effects of Intracranial Volume Correction Methods on Sex Differences in Neuroanatomical Structures: A HUNT MRI Study.” Frontiers in Neuroscience 9: 238. 10.3389/fnins.2015.00238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polanczyk, G. , de Lima M. S., Horta B. L., Biederman J., and Rohde L. A.. 2007. “The Worldwide Prevalence of ADHD: A Systematic Review and Metaregression Analysis.” American Journal of Psychiatry 164: 942–948. 10.1176/ajp.2007.164.6.942. [DOI] [PubMed] [Google Scholar]
- Potter, A. S. , Dube S. L., Barrios L. C., et al. 2022. “Measurement of Gender and Sexuality in the Adolescent Brain Cognitive Development (ABCD) Study.” Developmental Cognitive Neuroscience 53: 101057. 10.1016/j.dcn.2022.101057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potvin, O. , Mouiha A., Dieumegarde L., and Duchesne S.. 2018. “Corrigendum to ‘Normative Data for Subcortical Regional Volumes Over the Lifetime of the Adult Human Brain’ [NeuroImage 137 (2016) 9–20].” NeuroImage 183: 9–20. 10.1016/j.neuroimage.2018.09.020. [DOI] [PubMed] [Google Scholar]
- Potvin, O. , Mouiha A., Dieumegarde L., Duchesne S., and Alzheimer's Disease Neuroimaging Initiative . 2016. “Normative Data for Subcortical Regional Volumes Over the Lifetime of the Adult Human Brain.” NeuroImage 137: 9–20. 10.1016/j.neuroimage.2016.05.016. [DOI] [PubMed] [Google Scholar]
- Qing, Z. , and Gong G.. 2016. “Size Matters to Function: Brain Volume Correlates With Intrinsic Brain Activity Across Healthy Individuals.” NeuroImage 139: 271–278. 10.1016/j.neuroimage.2016.06.046. [DOI] [PubMed] [Google Scholar]
- Rauch, J. M. , and Eliot L.. 2022. “Breaking the Binary: Gender Versus Sex Analysis in Human Brain Imaging.” NeuroImage 264: 119732. 10.1016/j.neuroimage.2022.119732. [DOI] [PubMed] [Google Scholar]
- Rilling, J. K. , Lee M., Zhou C., Hepburn K., Perkins M. M., and Gaser C.. 2025. “Caregiving is Associated With Lower Brain Age in Humans.” Social Cognitive and Affective Neuroscience 20: nsaf013. 10.1093/scan/nsaf013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ringo, J. L. , Doty R. W., Demeter S., and Simard P. Y.. 1994. “Time Is of the Essence: A Conjecture That Hemispheric Specialization Arises From Interhemispheric Conduction Delay.” Cerebral Cortex 4: 331–343. 10.1093/cercor/4.4.331. [DOI] [PubMed] [Google Scholar]
- Rippon, G. , Jordan‐Young R., Kaiser A., and Fine C.. 2014. “Recommendations for Sex/Gender Neuroimaging Research: Key Principles and Implications for Research Design, Analysis, and Interpretation.” Frontiers in Human Neuroscience 8: 650. 10.3389/fnhum.2014.00650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritchie, S. J. , Cox S. R., Shen X., et al. 2018. “Sex Differences in the Adult Human Brain: Evidence From 5216 UK Biobank Participants.” Cerebral Cortex 28, no. 8: 2959–2975. 10.1093/cercor/bhy109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy, K. , and Chaudhuri A.. 2008. “Influence of Socioeconomic Status, Wealth and Financial Empowerment on Gender Differences in Health and Healthcare Utilization in Later Life: Evidence From India.” Social Science & Medicine 66: 1951–1962. 10.1016/j.socscimed.2008.01.015. [DOI] [PubMed] [Google Scholar]
- Rutter, M. , Caspi A., Fergusson D., et al. 2004. “Sex Differences in Developmental Reading Disability: New Findings From 4 Epidemiological Studies.” Journal of the American Medical Association 291: 2007–2012. 10.1001/jama.291.16.2007. [DOI] [PubMed] [Google Scholar]
- Ryali, S. , Zhang Y., de Los Angeles C., Supekar K., and Menon V.. 2024. “Deep Learning Models Reveal Replicable, Generalizable, and Behaviorally Relevant Sex Differences in Human Functional Brain Organization.” Proceedings of the National Academy of Sciences of the United States of America 121: e2310012121. 10.1073/pnas.2310012121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salk, R. H. , Hyde J. S., and Abramson L. Y.. 2017. “Gender Differences in Depression in Representative National Samples: Meta‐Analyses of Diagnoses and Symptoms.” Psychological Bulletin 143: 783–822. 10.1037/bul0000102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchis‐Segura, C. , Aguirre N., Cruz‐Gómez Á. J., Félix S., and Forn C.. 2022. “Beyond ‘Sex Prediction’: Estimating and Interpreting Multivariate Sex Differences and Similarities in the Brain.” NeuroImage 257: 119343. 10.1016/j.neuroimage.2022.119343. [DOI] [PubMed] [Google Scholar]
- Sanchis‐Segura, C. , Ibañez‐Gual M. V., Aguirre N., Cruz‐ Gómez Á. J., and Forn C.. 2020. “Effects of Different Intracranial Volume Correction Methods on Univariate Sex Differences in Grey Matter Volume and Multivariate Sex Prediction.” Scientific Reports 10: 1–15. 10.1038/s41598-020-69361-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sepehrband, F. , Lynch K. M., Cabeen R. P., et al. 2018. “Neuroanatomical Morphometric Characterization of Sex Differences in Youth Using Statistical Learning.” NeuroImage 172: 217–227. 10.1016/j.neuroimage.2018.01.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shanmugan, S. , Seidlitz J., Cui Z., et al. 2022. “Sex Differences in the Functional Topography of Association Networks in Youth.” Proceedings of the National Academy of Sciences of the United States of America 119: e2110416119. 10.1073/pnas.2110416119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaywitz, B. A. , Shaywitz S. E., Pugh K. R., et al. 1995. “Sex Differences in the Functional Organization of the Brain for Language.” Nature 373, no. 6515: 607–609. 10.1038/373607a0. [DOI] [PubMed] [Google Scholar]
- Smith, E. S. , Junger J., Derntl B., and Habel U.. 2015. “The Transsexual Brain–A Review of Findings on the Neural Basis of Transsexualism.” Neuroscience & Biobehavioral Reviews 59: 251–266. 10.1016/j.neubiorev.2015.09.008. [DOI] [PubMed] [Google Scholar]
- Sommer, I. E. , Aleman A., Somers M., Boks M. P., and Kahn R. S.. 2008. “Sex Differences in Handedness, Asymmetry of the Planum Temporale and Functional Language Lateralization.” Brain Research 1206: 76–88. 10.1016/j.brainres.2008.01.003. [DOI] [PubMed] [Google Scholar]
- Stewart‐Williams, S. , and Halsey L. G.. 2021. “Men, Women and STEM: Why the Differences and What Should Be Done?” European Journal of Personality 35: 3–39. 10.1177/0890207020962326. [DOI] [Google Scholar]
- Suo, C. , León I., Brodaty H., et al. 2012. “Supervisory Experience at Work Is Linked to Low Rate of Hippocampal Atrophy in Late Life.” NeuroImage 63: 1542–1551. 10.1016/j.neuroimage.2012.08.015. [DOI] [PubMed] [Google Scholar]
- Takao, H. , Hayashi N., and Ohtomo K.. 2014. “Sex Dimorphism in the White Matter: Fractional Anisotropy and Brain Size.” Journal of Magnetic Resonance Imaging 39: 917–923. 10.1002/jmri.24225. [DOI] [PubMed] [Google Scholar]
- Tan, A. , Ma W., Vira A., Marwha D., and Eliot L.. 2016. “The Human Hippocampus Is Not Sexually‐Dimorphic: Meta‐Analysis of Structural MRI Volumes.” NeuroImage 124: 350–366. 10.1016/j.neuroimage.2015.08.050. [DOI] [PubMed] [Google Scholar]
- Torgerson, C. , Ahmadi H., Choupan J., Fan C. C., Blosnich J. R., and Herting M. M.. 2024. “Sex, Gender Diversity, and Brain Structure in Early Adolescence.” Human Brain Mapping 45: e26671. 10.1002/hbm.26671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tozzi, L. , Zhang X., Pines A., et al. 2024. “Personalized Brain Circuit Scores Identify Clinically Distinct Biotypes in Depression and Anxiety.” Nature Medicine 30: 2076–2087. 10.1038/s41591-024-03057-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- US National Academies of Sciences, Engineering, and Medicine . 2022. Measuring Sex, Gender Identity, and Sexual Orientation. National Academies Press. 10.17226/26424. [DOI] [Google Scholar]
- van Anders, S. M. , and Watson N. V.. 2006. “Social Neuroendocrinology: Effects of Social Contexts and Behaviors on Sex Steroids in Humans.” Human Nature 17: 212–237. 10.1007/s12110-006-1018-7. [DOI] [PubMed] [Google Scholar]
- Vosberg, D. E. , Syme C., Parker N., Richer L., Pausova Z., and Paus T.. 2021. “Sex Continuum in the Brain and Body During Adolescence and Psychological Traits.” Nature Human Behavior 5: 265–272. 10.1038/s41562-020-00968-8. [DOI] [PubMed] [Google Scholar]
- Voyer, D. 2011. “Sex Differences in Dichotic Listening.” Brain and Cognition 76: 245–255. 10.1016/j.bandc.2011.02.001. [DOI] [PubMed] [Google Scholar]
- White, J. , Tannenbaum C., Klinge I., Schiebinger L., and Clayton J.. 2021. “The Integration of Sex and Gender Considerations Into Biomedical Research: Lessons From International Funding Agencies.” Journal of Clinical Endocrinology and Metabolism 106: 3034–3048. 10.1210/clinem/dgab434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wierenga, L. M. , Ruigrok A., Aksnes E. R., et al. 2023. “Recommendations for a Better Understanding of Sex and Gender in Neuroscience of Mental Health.” Biological Psychiatry Global Open Science 4: 100283. 10.1016/j.bpsgos.2023.100283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiersch, L. , Hamdan S., Hoffstaedter F., et al. 2023. “Accurate Sex Prediction of Cisgender and Transgender Individuals Without Brain Size Bias.” Scientific Reports 13, no. 1: 13868. 10.1038/s41598-023-37508-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams, C. M. , Peyre H., Toro R., and Ramus F.. 2021. “Neuroanatomical Norms in the UK Biobank: The Impact of Allometric Scaling, Sex, and Age.” Human Brain Mapping 42: 4623–4642. 10.1002/hbm.25572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams, C. M. , Peyre H., Toro R., and Ramus F.. 2022. “Comparing Brain Asymmetries Independently of Brain Size.” NeuroImage 254: 119118. 10.1016/j.neuroimage.2022.119118. [DOI] [PubMed] [Google Scholar]
- Yang, G. , Zhou S., Bozek J., et al. 2020. “Sample Sizes and Population Differences in Brain Template Construction.” NeuroImage 206: 116318. 10.1016/j.neuroimage.2019.116318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, C. , Dougherty C. C., Baum S. A., White T., and Michael A. M.. 2018. “Functional Connectivity Predicts Gender: Evidence for Gender Differences in Resting Brain Connectivity.” Human Brain Mapping 39: 1765–1776. 10.1002/hbm.23950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, Y. , Luo Q., Huang C.‐C., et al. 2021. “The Human Brain Is Best Described as Being on a Female/Male Continuum: Evidence From a Neuroimaging Connectivity Study.” Cerebral Cortex 31: 3021–3033. 10.1093/cercor/bhaa408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou, J. N. , Hofman M. A., Gooren L. J., and Swaab D. F.. 1995. “A Sex Difference in the Human Brain and Its Relation to Transsexuality.” Nature 378: 68–70. 10.1038/378068a0. [DOI] [PubMed] [Google Scholar]
- Zirra, A. , Rao S. C., Bestwick J., et al. 2023. “Gender Differences in the Prevalence of Parkinson's Disease.” Movement Disorders Clinical Practice 10: 86–93. 10.1002/mdc3.13584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou, J. , and Schiebinger L.. 2018. “AI can be Sexist and Racist — it’s Time to Make it Fair.” Nature 559: 324–326. 10.1038/d41586-018-05707-8.. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing not applicable to this article as no datasets were generated or analysed during the current study.


