Abstract
1. An α-(1→6)-glucosidase has been separated from cell extracts of Streptococcus mitis. The enzyme was freed from transglucosylase by adsorption of the latter on retrograded amylose. 2. The enzyme was detected in five of the six strains of S. mitis that were studied; α-(1→6)-glucosidase was not found in strain RB1633, a strain that did not store polysaccharide. 3. The glucosidase could act on compounds in which α-glucose is joined through an α-(1→6)-bond to either a maltosaccharide or an isomaltosaccharide. 62-α-Glucosylmaltose (panose) and 63-α-glucosylmaltotriose were hydrolysed more rapidly and isomaltodextrins more slowly than isomaltose. 4. Transferring activity towards isomaltose and panose was appreciable when the concentration of substrate was 2% or higher. 5. The enzyme had no action on α-(1→4)-glucosidic linkages. 6-α-Maltodextrinylglucoses were hydrolysed only after transglucosylase action had attenuated them to isomaltose.
Full text
PDF
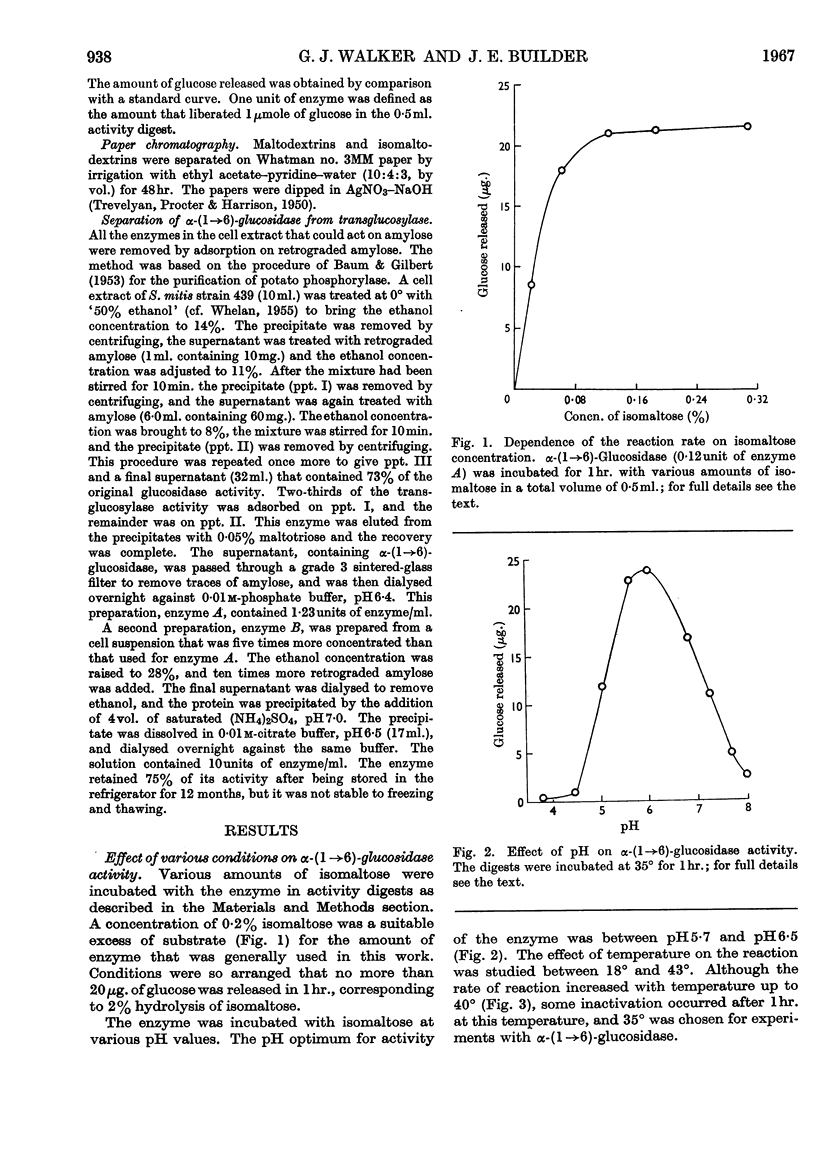
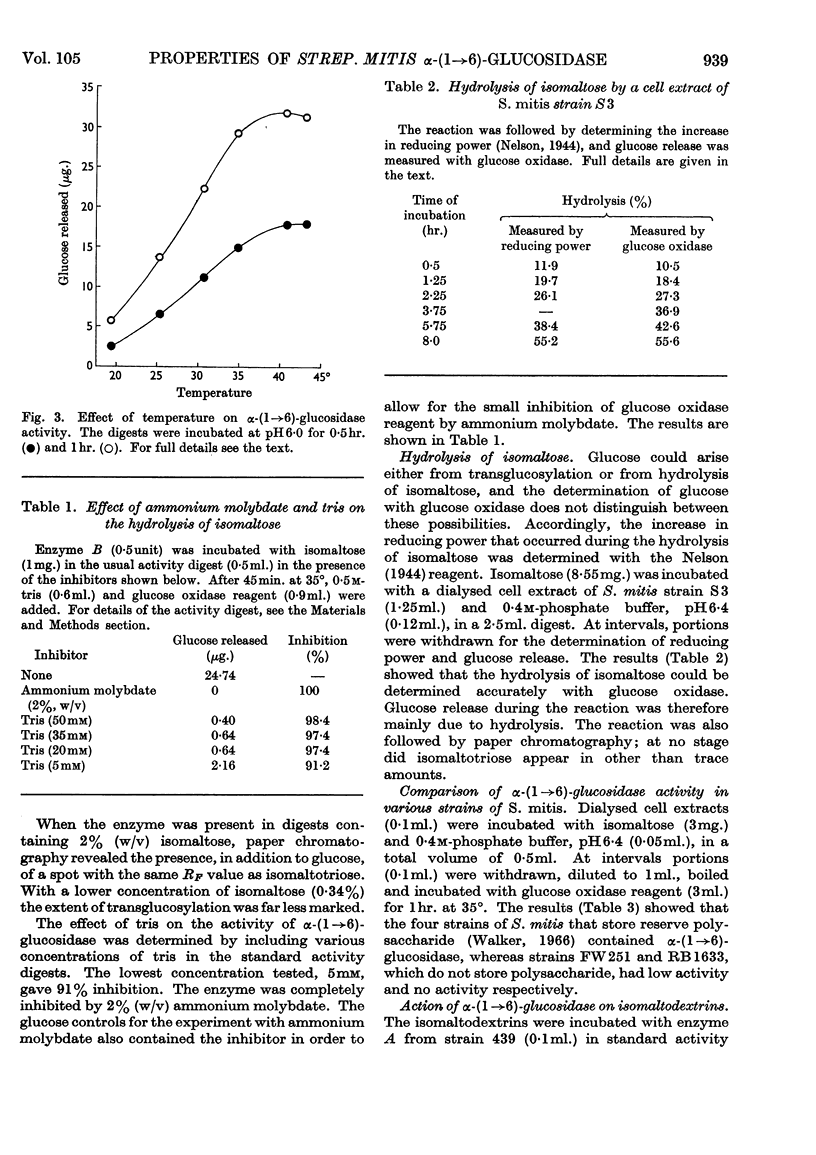
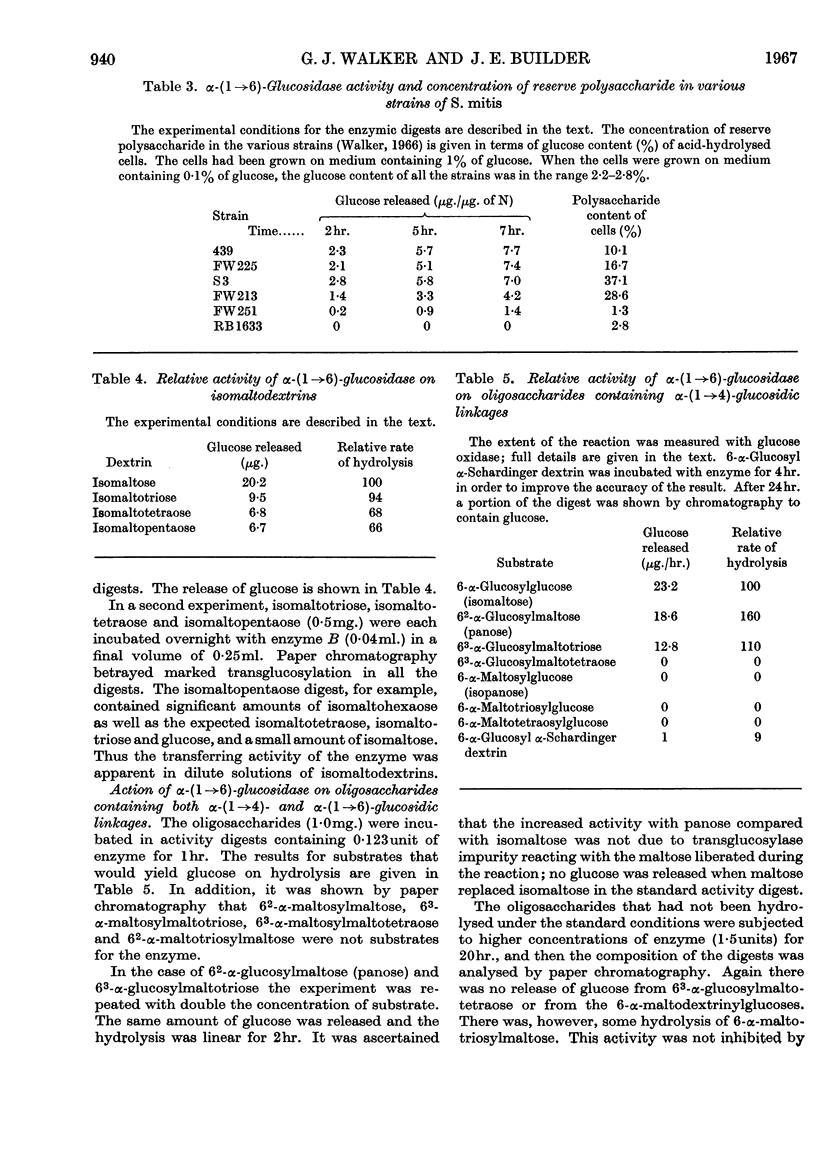


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BAILEY R. W., ROBERTON A. M. Carbohydrases of a rumen strain of Lactobacillus bifidus. 2. The intracellular alpha-1-6-glucosidase (isomaltodextrinase). Biochem J. 1962 Feb;82:272–277. doi: 10.1042/bj0820272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BAUM H., GILBERT G. A. A simple method for the preparation of crystalline potato phosphorylase and Q-enzyme. Nature. 1953 May 30;171(4361):983–984. doi: 10.1038/171983a0. [DOI] [PubMed] [Google Scholar]
- DAHLQVIST A. Determination of maltase and isomaltase activities with a glucose-oxidase reagent. Biochem J. 1961 Sep;80:547–551. doi: 10.1042/bj0800547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAHLQVIST A. Rat-intestinal dextranase. Localization and relation to the other carbohydrases of the digestive tract. Biochem J. 1963 Jan;86:72–76. doi: 10.1042/bj0860072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GIBBONS R. J., KAPSIMALIS B. Synthesis of intracellular iodophilic polysaccharide by Streptococcus mitis. Arch Oral Biol. 1963 May-Jun;8:319–329. doi: 10.1016/0003-9969(63)90024-4. [DOI] [PubMed] [Google Scholar]
- Illingworth B., Brown D. H. ACTION OF AMYLO-1,6-GLUCOSIDASE ON LOW MOLECULAR WEIGHT SUBSTRATES AND THE ASSAY OF THIS ENZYME IN GLYCOGEN STORAGE DISEASE. Proc Natl Acad Sci U S A. 1962 Sep;48(9):1619–1623. doi: 10.1073/pnas.48.9.1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KORN E. D., NORTHCOTE D. H. Physical and chemical properties of polysaccharides and glycoproteins of the yeast-cell wall. Biochem J. 1960 Apr;75:12–17. doi: 10.1042/bj0750012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LARNER J., McNICKLE C. M. Gastrointestinal digestion of starch. I. The action of oligo-1,6-glucosidase on branched saccharides. J Biol Chem. 1955 Aug;215(2):723–736. [PubMed] [Google Scholar]
- PAZUR J. H., ANDO T. The hydrolysis of glucosyl oligosaccharides with alpha-D-(1-4) and alpha-D-(1-6) bonds by fungal amyloglucosidase. J Biol Chem. 1960 Feb;235:297–302. [PubMed] [Google Scholar]
- TREVELYAN W. E., PROCTER D. P., HARRISON J. S. Detection of sugars on paper chromatograms. Nature. 1950 Sep 9;166(4219):444–445. doi: 10.1038/166444b0. [DOI] [PubMed] [Google Scholar]
- Taylor P. M., Whelan W. J. A new substrate for the detection of amylo-1,6-glucosidase, and its application to the study of glycogen-storage disease type 3. Arch Biochem Biophys. 1966 Feb;113(2):500–502. doi: 10.1016/0003-9861(66)90221-9. [DOI] [PubMed] [Google Scholar]
- Walker G. J. Metabolism of the reserve polysaccharide of Streptococcus mitis: Properties of a transglucosylase. Biochem J. 1966 Dec;101(3):861–872. doi: 10.1042/bj1010861. [DOI] [PMC free article] [PubMed] [Google Scholar]


