Abstract
1. The partition of uroporphyrins I and III, coproporphyrins I and III, haematoporphyrin IX, porphyrin c and a hydrophilic porphyrin–peptide fraction from variegate-porphyria faeces has been studied in systems of equal volumes of cyclohexanone and sodium acetate buffers of varying pH and concentration. 2. The concentration of acetate in the aqueous phase has little effect on the partition of porphyrin c, but markedly influences that of uroporphyrin. At 50% acetate saturation and pH4·5, only 5% enters the cyclohexanone phase whereas 60% of porphyrin c is extracted under similar conditions. 3. This circumstance forms the basis of a method for the determination of hydrophilic porphyrin–peptides in variegate-porphyria urine. Its reliability has been checked in model experiments. 4. At pH1·5 and an aqueous phase half-saturated with sodium acetate, an equal volume of cyclohexanone removes 95–97% of uroporphyrin and about 55% of porphyrin c. Uroporphyrin may therefore be determined as a second step in the method. 5. For the routine determination of uroporphyrin in systems free from other hydrophilic porphyrins, cyclohexanone extraction may be performed at any pH in the range 1·0–3·0.
Full text
PDF

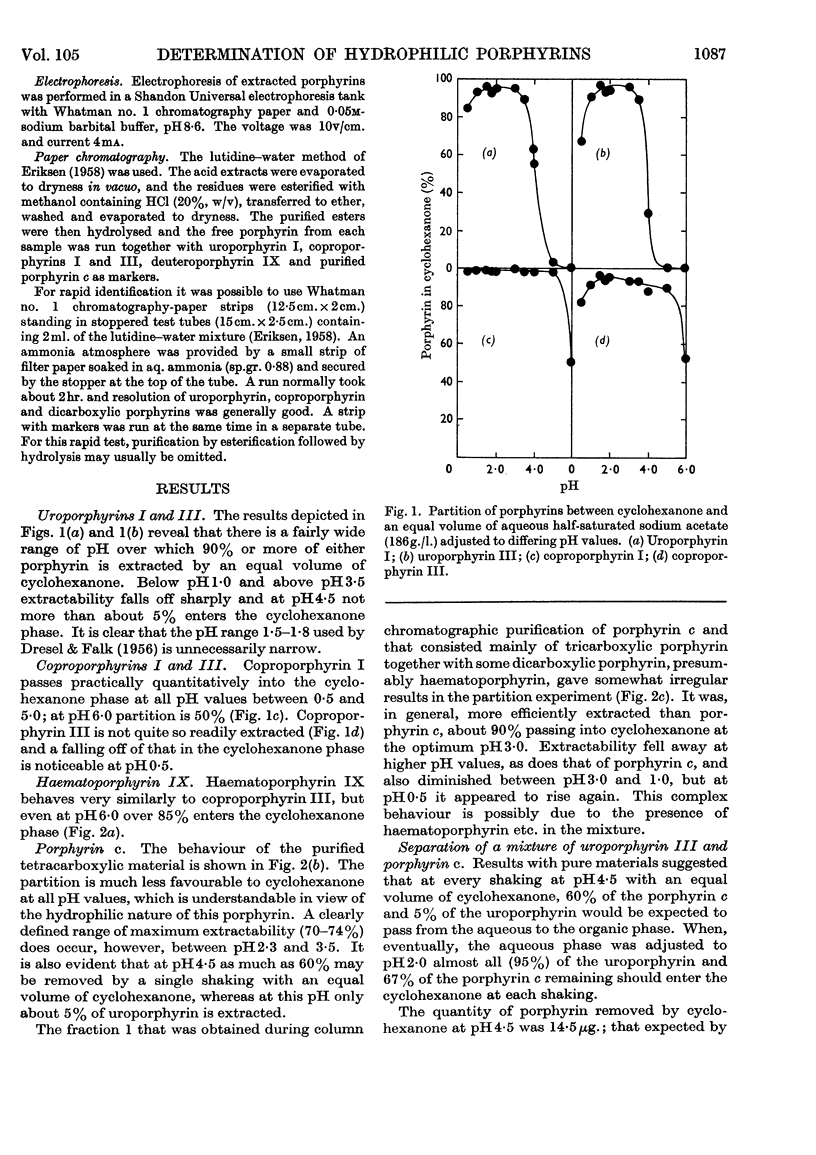

Selected References
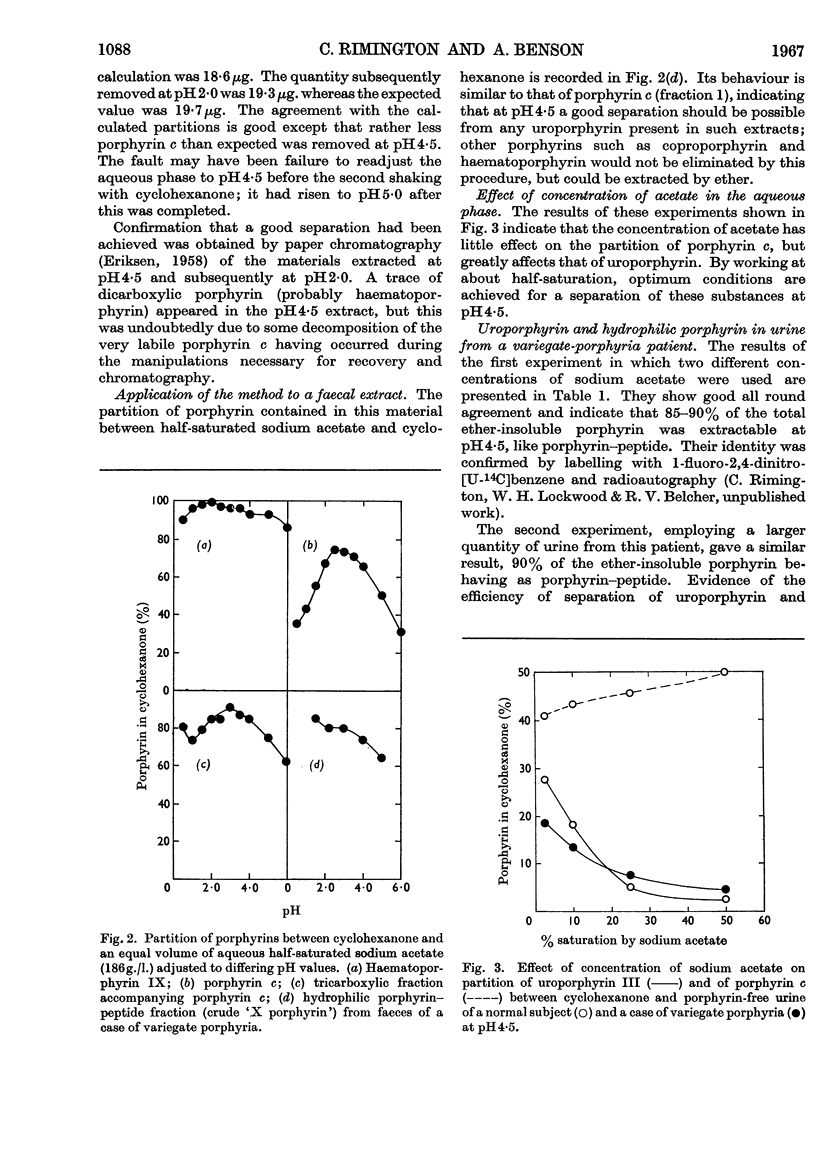
These references are in PubMed. This may not be the complete list of references from this article.
- DRESEL E. I., FALK J. E. Studies on the biosynthesis of blood pigments. 2. Haem and porphyrin formation in intact chicken erythrocytes. Biochem J. 1956 May;63(1):72–79. doi: 10.1042/bj0630072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DRESEL E. I., RIMINGTON C., TOOTH B. E. Determination of urinary uroporphyrin by a direct extraction method. Scand J Clin Lab Invest. 1956;8(1):73–78. doi: 10.3109/00365515609049247. [DOI] [PubMed] [Google Scholar]
- DRESEL E. I., TOOTH B. E. Solubility of uroporphyrin I in ethyl acetate. Nature. 1954 Aug 7;174(4423):271–271. doi: 10.1038/174271a0. [DOI] [PubMed] [Google Scholar]
- ERIKSEN L. Paper chromatographic separation of the coproporphyrin isomers I and III. Scand J Clin Lab Invest. 1958;10(3):319–322. [PubMed] [Google Scholar]
- Gray C. H. The isolation of coproporphyrin III from Corynebacterium diphtheriae culture filtrates. Biochem J. 1948;43(2):191–193. [PMC free article] [PubMed] [Google Scholar]
- Gray C. H. The isolation of coproporphyrin III from Corynebacterium diphtheriae culture filtrates. Biochem J. 1948;43(2):191–193. [PMC free article] [PubMed] [Google Scholar]
- RIMINGTON C., MILES P. A. A study of the porphyrins excreted in the urine by a case of congenital porphyria. Biochem J. 1951 Dec;50(2):202–206. doi: 10.1042/bj0500202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RIMINGTON C., SVEINSSON S. L. The spectrophotometric determination of uroporphyrin. Scand J Clin Lab Invest. 1950;2(3):209–216. doi: 10.3109/00365515009049872. [DOI] [PubMed] [Google Scholar]
- Rimington C. Spectral-absorption coefficients of some porphyrins in the Soret-band region. Biochem J. 1960 Jun;75(3):620–623. doi: 10.1042/bj0750620. [DOI] [PMC free article] [PubMed] [Google Scholar]


