Abstract
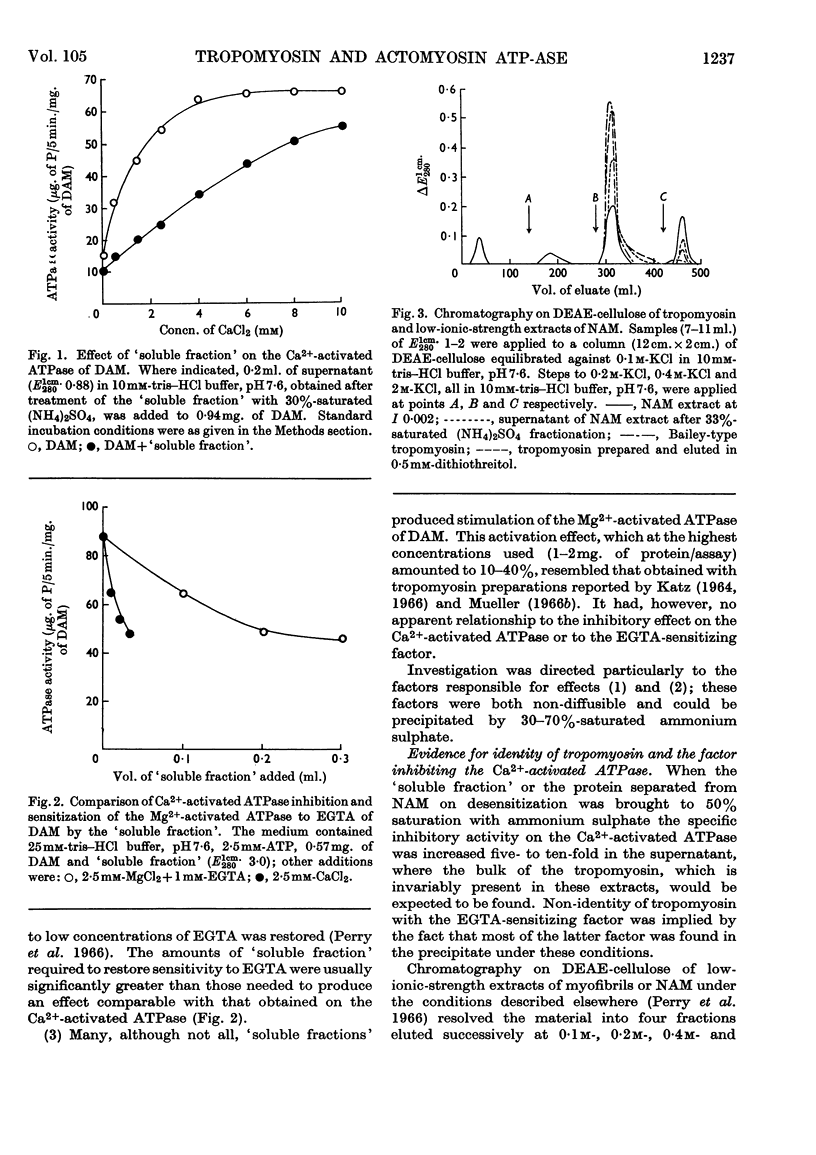
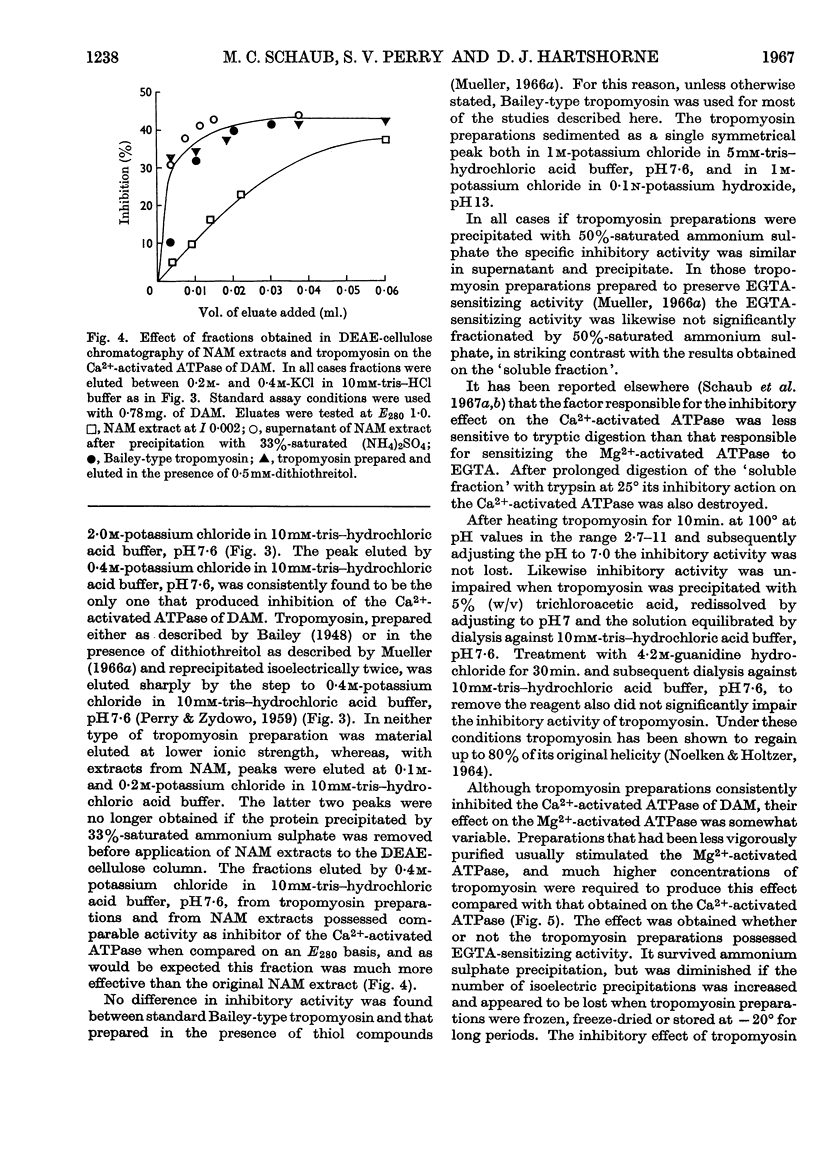
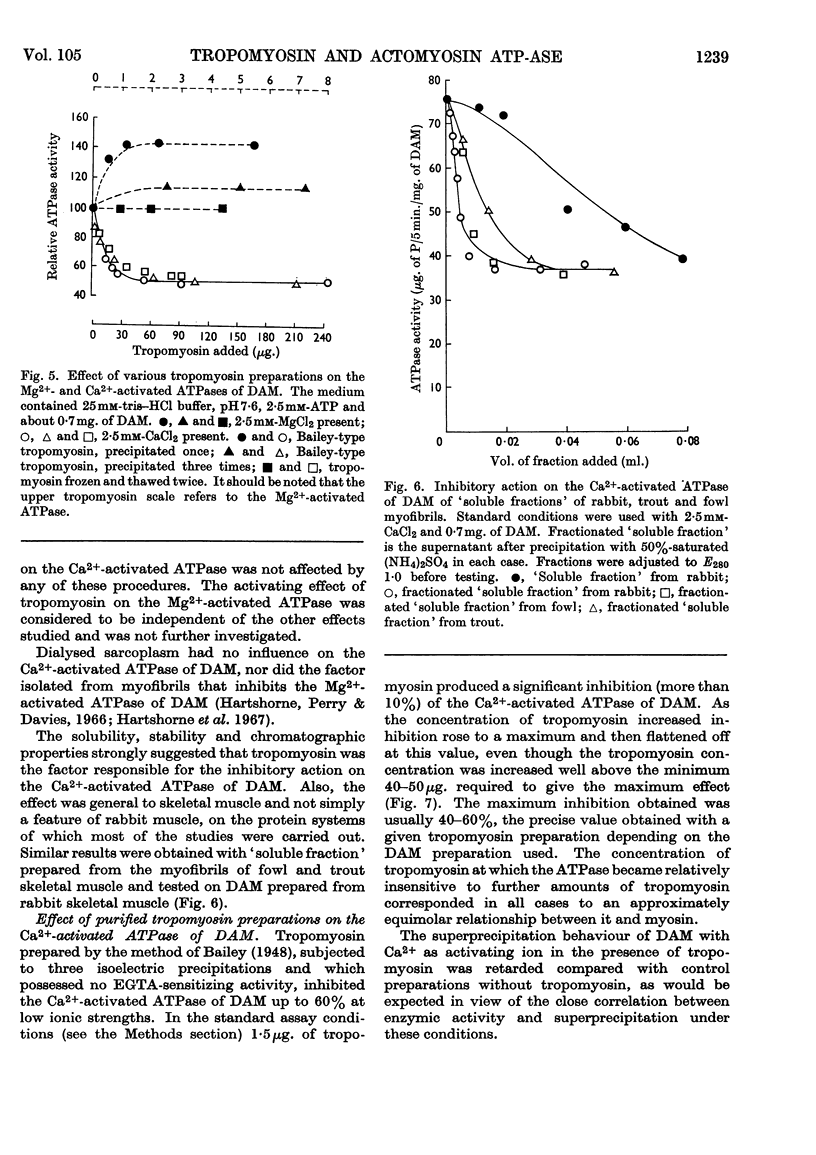
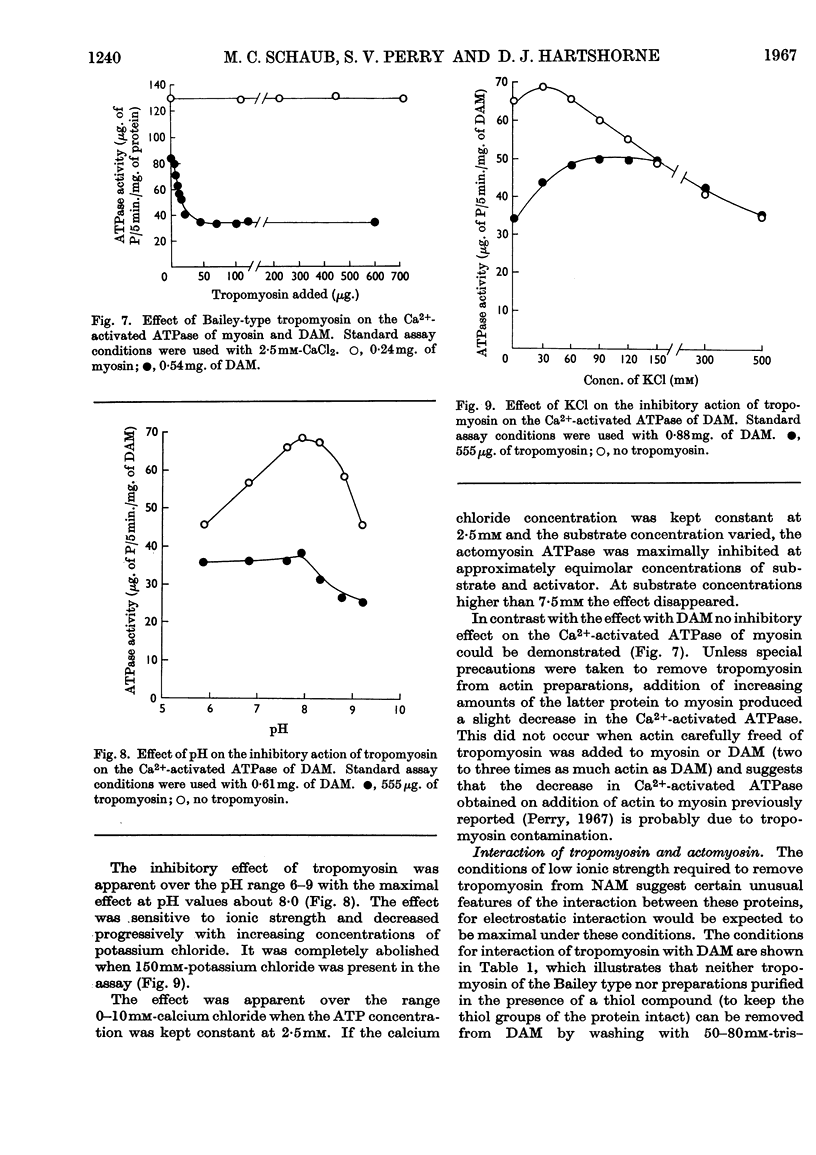
1. Tropomyosin preparations of the Bailey type, and those prepared in the presence of dithiothreitol to prevent oxidation of protein thiol groups, inhibit the Ca2+-activated adenosine triphosphatase (ATPase) of desensitized actomyosin by up to 60%. 2. The inhibitory activity of myofibrillar extracts and tropomyosin survives various agents known to denature proteins but to the action of which tropomyosin is unusually stable, namely heating at 100° and mild tryptic digestion. It is destroyed by prolonged treatment with trypsin. 3. The ethylenedioxybis-(ethyleneamino)tetra-acetic acid (EGTA)-sensitizing factor present in extracts of natural actomyosin and myofibrils could be selectively destroyed, leaving unchanged the inhibitory effect on the Ca2+-activated ATPase. There was no correlation between the EGTA-sensitizing and the Ca2+-activated inhibitory activities of tropomyosin prepared under different conditions. 4. Optimum inhibition was achieved when tropomyosin and the myosin of desensitized actomyosin were present in approximately equimolar proportions. Tropomyosin had no effect on the Ca2+-activated ATPase of myosin measured under similar conditions. 5. Evidence is presented showing that the tropomyosin binds to desensitized actomyosin under the conditions in which the ATPase is inhibited.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bailey K. Tropomyosin: a new asymmetric protein component of the muscle fibril. Biochem J. 1948;43(2):271–279. doi: 10.1042/bj0430271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EBASHI S. THIRD COMPONENT PARTICIPATING IN THE SUPERPRECIPITATION OF 'NATURAL ACTOMYOSIN'. Nature. 1963 Dec 7;200:1010–1010. doi: 10.1038/2001010a0. [DOI] [PubMed] [Google Scholar]
- Hartshorne D. J., Mueller H. Separation and recombination of the ethylene glycol bis (beta-aminoethyl ether)-N,N'-tetraacetic acid-sensitizing factor obtained from a low ionic strength extract of natural actomyosin. J Biol Chem. 1967 Jul 10;242(13):3089–3092. [PubMed] [Google Scholar]
- Hartshorne D. J., Perry S. V., Davies V. A factor inhibiting the adenosine triphosphatase activity and the superprecipitation of actomyosin. Nature. 1966 Mar 26;209(5030):1352–1353. doi: 10.1038/2091352a0. [DOI] [PubMed] [Google Scholar]
- Hartshorne D. J., Perry S. V., Schaub M. C. A protein factor inhibiting the magnesium-activated adenosine triphosphatase of desensitized actomyosin. Biochem J. 1967 Sep;104(3):907–913. doi: 10.1042/bj1040907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson P., Harris C. I., Perry S. V. 3-methylhistidine in actin and other muscle proteins. Biochem J. 1967 Oct;105(1):361–370. doi: 10.1042/bj1050361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KATZ A. M. INFLUENCE OF TROPOMYOSIN UPON THE REACTIONS OF ACTOMYOSIN AT LOW IONIC STRENGTH. J Biol Chem. 1964 Oct;239:3304–3311. [PubMed] [Google Scholar]
- Katz A. M. Purification and properties of a tropomyosin-containing protein fraction that sensitizes reconstituted actomyosin to calcium-binding agents. J Biol Chem. 1966 Apr 10;241(7):1522–1529. [PubMed] [Google Scholar]
- Kominz D. R., Maruyama K. Does native tropomyosin bind to myosin? J Biochem. 1967 Feb;61(2):269–271. doi: 10.1093/oxfordjournals.jbchem.a128542. [DOI] [PubMed] [Google Scholar]
- LAKI K., MARUYAMA K., KOMINZ D. R. Evidence for the interaction between tropomyosin and actin. Arch Biochem Biophys. 1962 Aug;98:323–330. doi: 10.1016/0003-9861(62)90190-x. [DOI] [PubMed] [Google Scholar]
- LEADBEATER L., PERRY S. V. The effect of actin on the magnesium-activated adenosine triphosphatase of heavy meromyosin. Biochem J. 1963 May;87:233–239. doi: 10.1042/bj0870233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARTONOSI A. Studies on actin. VII. Ultracentrifugal analysis of partially polymerized actin solutions. J Biol Chem. 1962 Sep;237:2795–2803. [PubMed] [Google Scholar]
- MARUYAMA K. INTERACTION OF TROPOMYOSIN WITH ACTIN. A FLOW BIREFRINGENCE STUDY. Arch Biochem Biophys. 1964 Apr;105:142–150. doi: 10.1016/0003-9861(64)90245-0. [DOI] [PubMed] [Google Scholar]
- Mueller H. Effect of Bailey's tropomyosin purification on EGTA-sensitizing activity. Nature. 1966 Mar 12;209(5028):1128–1129. doi: 10.1038/2091128a0. [DOI] [PubMed] [Google Scholar]
- PERRY S. V., CORSI A. Extraction of proteins other than myosin from the isolated rabbit myofibril. Biochem J. 1958 Jan;68(1):5–12. doi: 10.1042/bj0680005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PERRY S. V. The chromatography of L-myosin on diethylaminoethylcellulose. Biochem J. 1960 Jan;74:94–101. doi: 10.1042/bj0740094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PERRY S. V. The protein components of the isolated myofibril. Biochem J. 1953 Aug;55(1):114–122. doi: 10.1042/bj0550114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PERRY S. V., ZYDOWO M. The nature of the extra protein fraction from myofibrils of striated muscle. Biochem J. 1959 Feb;71(2):220–228. doi: 10.1042/bj0710220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry S. V., Davies V., Hayter D. Natural tropomyosin and the factor sensitizing actomyosin adenosine triphosphatase to ethylenedioxybis(ethyleneamino)tetra-acetic acid. Biochem J. 1966 Apr;99(1):1C–2C. doi: 10.1042/bj0990001c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHWARTZ A., BACHELARD H. S., McIL WAIN H. The sodium-stimulated adenosine-triphosphatase activity and other properties of cerebral microsomal fractions and subfractions. Biochem J. 1962 Sep;84:626–637. doi: 10.1042/bj0840626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaub M. C., Hartshorne D. J., Perry S. V. Effect of tropomyosin on the calcium-activated adenosine triphosphatase of actomyosin. Nature. 1967 Aug 5;215(5101):635–636. doi: 10.1038/215635a0. [DOI] [PubMed] [Google Scholar]
- Schaub M. C., Hartshorne D. J., Perry S. V. The adeonosine-triphosphatase activity of desensitized actomyosin. Biochem J. 1967 Jul;104(1):263–269. doi: 10.1042/bj1040263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe S., Staprans I. Purification of the relaxing protein of rabbit skeletal muscle. Proc Natl Acad Sci U S A. 1966 Aug;56(2):572–577. doi: 10.1073/pnas.56.2.572. [DOI] [PMC free article] [PubMed] [Google Scholar]


