Abstract
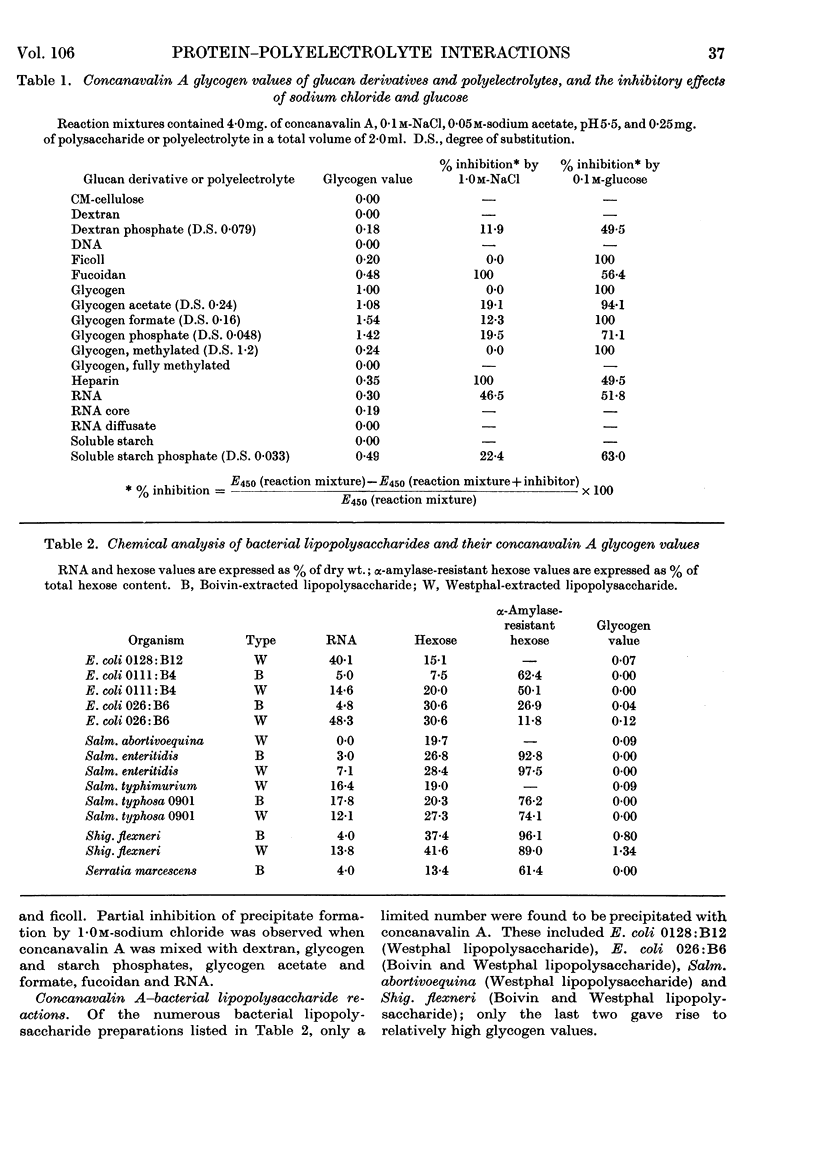
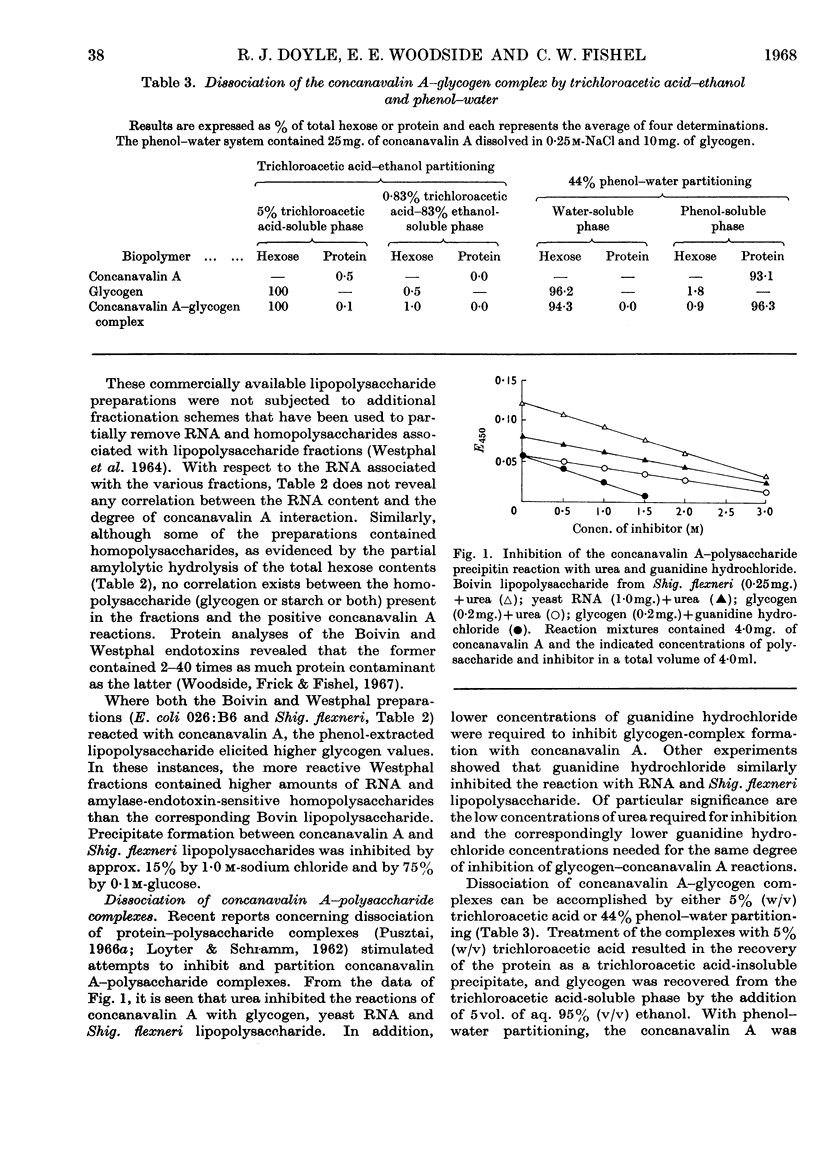
1. Concanavalin A formed precipitates with polyelectrolytes such as fucoidan, RNA, heparin and bacterial lipopolysaccharides. 2. Precipitate formation also occurred between ficoll and concanavalin A. 3. Precipitate formation between concanavalin A and dextran or soluble starch was induced by the incorporation of phosphate groups into the unreactive glucans. 4. Introduction of polar groups, such as acetate, formate and phosphate, into glycogen resulted in enhanced precipitation with concanavalin A, whereas the opposite effect was noted on incorporation of hydrophobic (methyl) centres. 5. Neutral sugars and salt partially inhibited complex-formation between polyelectrolytes and concanavalin A. 6. Concanavalin A–glycogen complexes could be dissociated with 5% (w/v) trichloroacetic acid or 44% phenol–water. 7. Concanavalin A lost its glycogen-complexing ability after phenol treatment. 8. Evidence is presented for the existence of common binding sites on concanavalin A for both neutral polysaccharides and polyelectrolytes. 9. Hydrogen bonding appeared to play a major role in neutral polysaccharide–concanavalin A precipitate formation, whereas both hydrogen bonding and electrostatic forces were implicated in polyelectrolyte–concanavalin A complex-formation.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agrawal B. B., Goldstein I. J. Physical and chemical characterization of concanavalin A, the hemagglutinin from jack bean (Canavalia ensiformis). Biochim Biophys Acta. 1967 Feb 21;133(2):376–379. doi: 10.1016/0005-2795(67)90081-5. [DOI] [PubMed] [Google Scholar]
- BANKS W. PHYSICAL PROPERTIES OF SOLUTIONS OF POLYSACCHARIDES. Adv Carbohydr Chem. 1963;18:357–398. doi: 10.1016/s0096-5332(08)60247-7. [DOI] [PubMed] [Google Scholar]
- Bello J., Haas D., Bello H. R. Interactions of protein-denaturing salts with model amides. Biochemistry. 1966 Aug;5(8):2539–2548. doi: 10.1021/bi00872a008. [DOI] [PubMed] [Google Scholar]
- Boyd W. C., Shapleigh E. Specific Precipitating Activity of Plant Agglutinins (Lectins). Science. 1954 Mar 26;119(3091):419–419. doi: 10.1126/science.119.3091.419. [DOI] [PubMed] [Google Scholar]
- GOLDSTEIN I. J., HOLLERMAN C. E., MERRICK J. M. PROTEIN-CARBOHYDRATE INTERACTION. I. THE INTERACTION OF POLYSACCHARIDES WITH CONCANAVALIN A. Biochim Biophys Acta. 1965 Jan 4;97:68–76. doi: 10.1016/0304-4165(65)90270-9. [DOI] [PubMed] [Google Scholar]
- GOLDSTEIN I. J., HOLLERMAN C. E., SMITH E. E. PROTEIN-CARBOHYDRATE INTERACTION. II. INHIBITION STUDIES ON THE INTERACTION OF CONCANAVALIN A WITH POLYSACCHARIDES. Biochemistry. 1965 May;4:876–883. doi: 10.1021/bi00881a013. [DOI] [PubMed] [Google Scholar]
- Goldstein I. J., Iyer R. N. Interaction of concanavalin A, a phytohemagglutinin, with model substrates. Biochim Biophys Acta. 1966 May 26;121(1):197–200. doi: 10.1016/0304-4165(66)90374-6. [DOI] [PubMed] [Google Scholar]
- Goldstein I. J., So L. L. Protein-carbonhydrate interaction. 3. Agar gel-diffusion studies on the interaction of Concanavalin A, a lectin isolated from jack bean, with polysaccharides. Arch Biochem Biophys. 1965 Aug;111(2):407–414. doi: 10.1016/0003-9861(65)90203-1. [DOI] [PubMed] [Google Scholar]
- HARRIS H., ROBSON E. B. Precipitin reactions between extracts of seeds of Canavalia ensiformis (Jack Bean) and normal and pathological serum proteins. Vox Sang. 1963 May-Jun;8:348–355. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- LOYTER A., SCHRAMM M. The glycogen-amylase complex as a means of obtaining highly purified alpha-amylases. Biochim Biophys Acta. 1962 Dec 4;65:200–206. doi: 10.1016/0006-3002(62)91039-9. [DOI] [PubMed] [Google Scholar]
- MOKRASCH L. C. Analysis of hexose phosphates and sugar mixtures with the anthrone reagent. J Biol Chem. 1954 May;208(1):55–59. [PubMed] [Google Scholar]
- Mellman W. J., Rawnsley H. M. Blastogenesis in peripheral blood lymphocytes in response to phytohemagglutinin and antigens. Fed Proc. 1966 Nov-Dec;25(6):1720–1722. [PubMed] [Google Scholar]
- NOWELL P. C. Phytohemagglutinin: an initiator of mitosis in cultures of normal human leukocytes. Cancer Res. 1960 May;20:462–466. [PubMed] [Google Scholar]
- Nakamura S., Suzuno R. Crystallization of concanavalins A and B and canavalin from Japanese jack beans. Arch Biochem Biophys. 1965 Sep;111(3):499–505. doi: 10.1016/0003-9861(65)90228-6. [DOI] [PubMed] [Google Scholar]
- Olson M. O., Liener I. E. Some physical and chemical properties of concanavalin A, the phytohemagglutinin of the jack bean. Biochemistry. 1967 Jan;6(1):105–111. doi: 10.1021/bi00853a018. [DOI] [PubMed] [Google Scholar]
- Pusztai A. Interactions of proteins with other polyelectrolytes in a two-phase system containing phenol and aqueous buffers at various pH values. Biochem J. 1966 Apr;99(1):93–101. doi: 10.1042/bj0990093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pusztai A. The properties of bovine serum albumin and chymotrypsinogen A in solvent mixtures containing phenol. Biochem J. 1966 Nov;101(2):265–273. doi: 10.1042/bj1010265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RIGAS D. A., OSGOOD E. E. Purification and properties of the phytohemagglutinin of Phaseolus vulgaris. J Biol Chem. 1955 Feb;212(2):607–615. [PubMed] [Google Scholar]
- ROBINSON D. R., JENCKS W. P. THE EFFECT OF COMPOUNDS OF THE UREA-GUANIDINIUM CLASS ON THE ACTIVITY COEFFICIENT OF ACETYLTETRAGLYCINE ETHYL ESTER AND RELATED COMPOUNDS. J Am Chem Soc. 1965 Jun 5;87:2462–2470. doi: 10.1021/ja01089a028. [DOI] [PubMed] [Google Scholar]
- WEBB J. M. A sensitive method for the determination of ribonucleic acid in tissues and microorganisms. J Biol Chem. 1956 Aug;221(2):635–649. [PubMed] [Google Scholar]
- WOODSIDE E. E., KOCHOLATY W. Carbohydrates of human and bovine platelets. Blood. 1960 Aug;16:1173–1183. [PubMed] [Google Scholar]
- Woodside E. E., Trott G. F., Doyle R. J., Fishel C. W. Polyglucose-protein complexes. Arch Biochem Biophys. 1966 Oct;117(1):125–133. [PubMed] [Google Scholar]


