Abstract
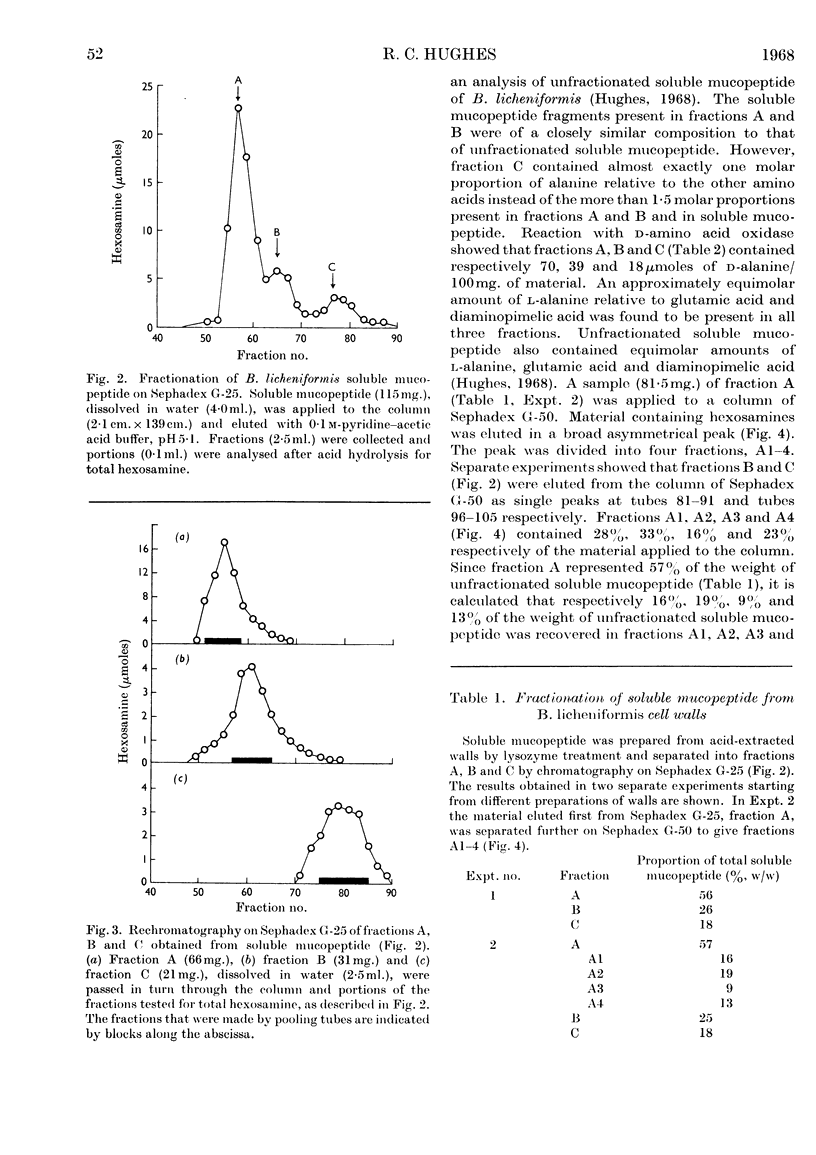
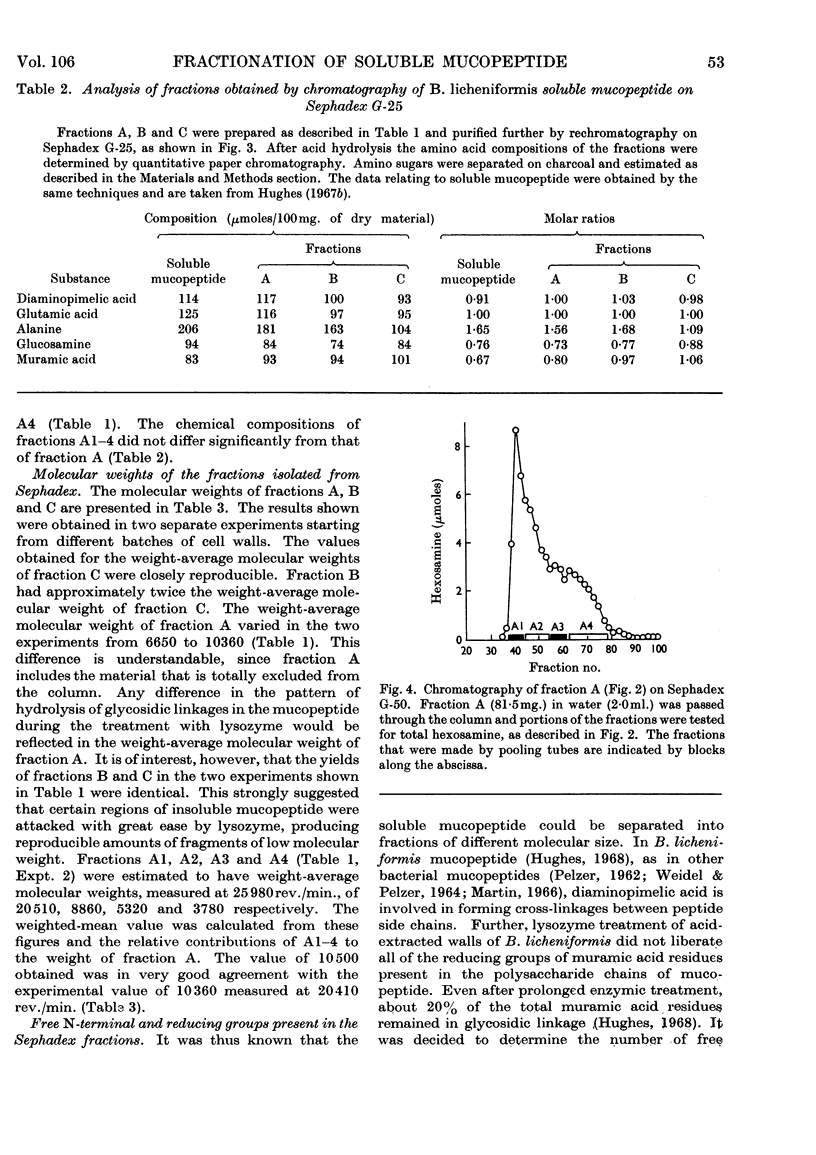
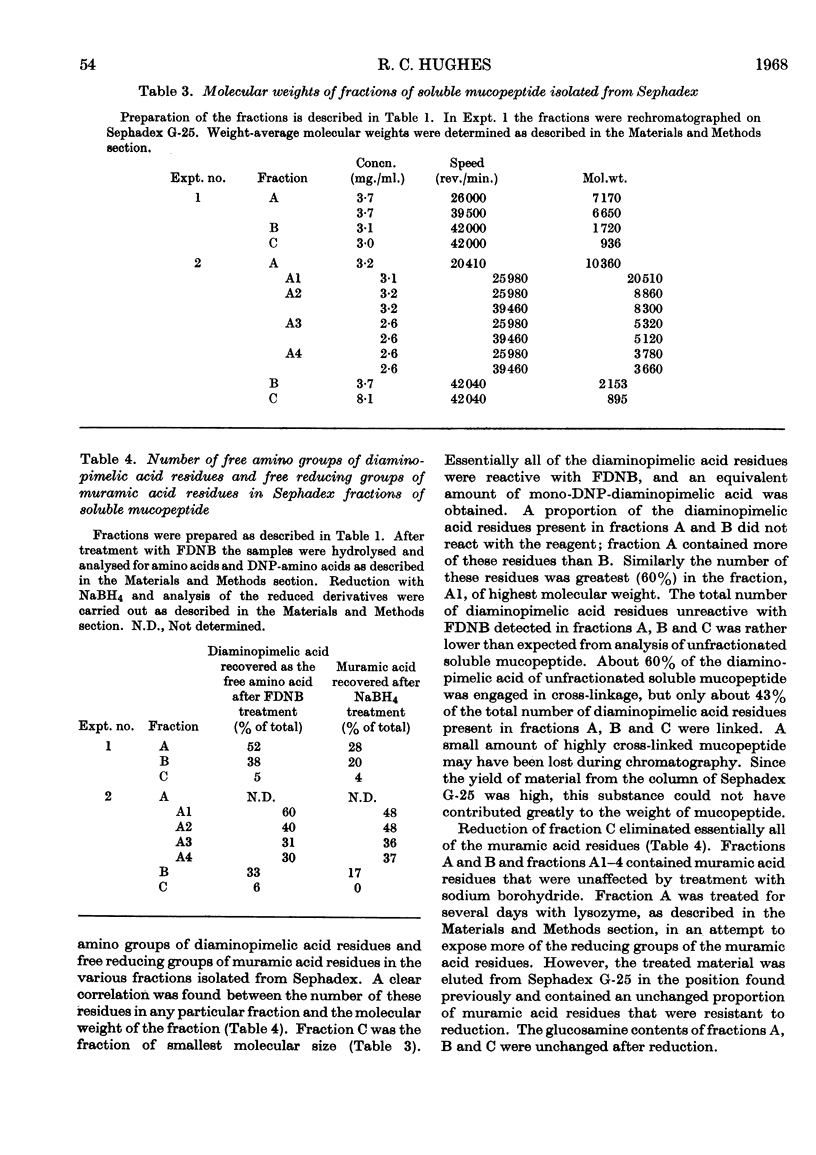
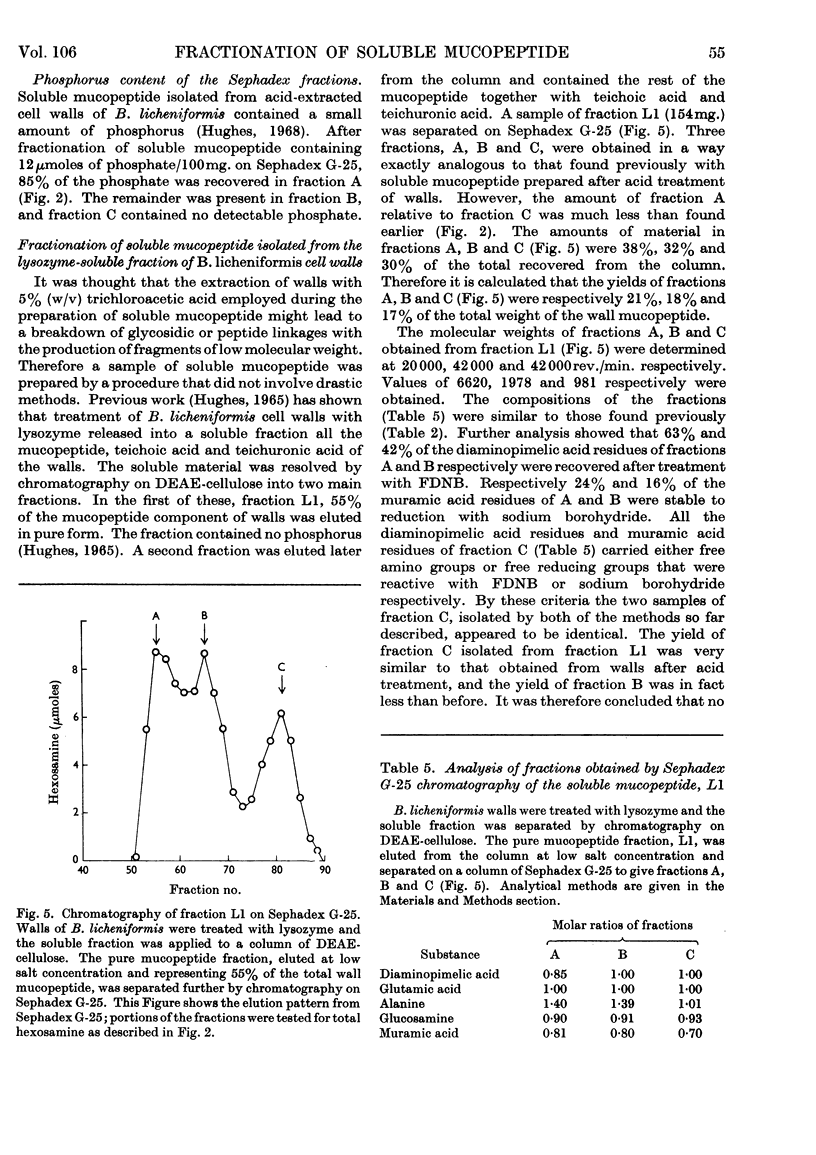
1. Soluble mucopeptide was prepared by lysozyme treatment of acid-extracted walls of Bacillus licheniformis N.C.T.C. 6346 and separated into fractions differing in molecular size by chromatography on Sephadex G-25 and G-50. 2. About 16% of the weight of soluble mucopeptide has a weight-average molecular weight in excess of 20000. About one half has a weight-average molecular weight of less than 2000 and the balance of soluble mucopeptide is of intermediate size. 3. In the mucopeptide fractions isolated from Sephadex there is a correlation between the weight-average molecular weight, the number of non-reducing muramic acid residues and the proportion of diaminopimelic acid residues recovered after treatment with 1-fluoro-2,4-dinitrobenzene. 4. The extent of cross-linking between peptide side chains is relatively low, even in mucopeptide material of the large molecular size. 5. The small amount of residual phosphorus present in preparations of B. licheniformis soluble mucopeptide remains associated mainly with mucopeptide material of large molecular size. 6. The mucopeptide components of lowest molecular weight are not produced as artifacts during the preparation of soluble mucopeptide, but are apparently incorporated in the insoluble mucopeptide present in walls of exponentially growing cells. 7. Soluble mucopeptide isolated in a complex with acidic polymers after lysozyme treatment of walls of B. licheniformis N.C.T.C. 6346 and Bacillus subtilis W23 retains a high molecular weight when the covalent bonds between mucopeptide and the acidic polymers are broken. 8. Pure fragments were isolated from B. licheniformis soluble mucopeptide. A major component, C1, of the material of smallest size is made up of one residue each of N-acetylglucosamine, N-acetylmuramic acid, l-alanine, glutamic acid and diaminopimelic acid. The N-acetylglucosamine is in β-glycosidic linkage with a reducing N-acetylmuramic acid residue. The peptide unit is probably amidated. A quantitatively minor component, C2, has amino acid and amino sugar composition identical with that of component C1, but probably lacks an amide group. Another fragment, B1, is made up of two molecules of component C1 or C2 that are joined together through a molecule of d-alanine.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BERGER L. R., WEISER R. S. The beta-glucosaminidase activity of egg-white lysozyme. Biochim Biophys Acta. 1957 Dec;26(3):517–521. doi: 10.1016/0006-3002(57)90098-7. [DOI] [PubMed] [Google Scholar]
- BRUMFITT W., WARDLAW A. C., PARK J. T. Development of lysozyme-resistance in Micrococcus lysodiekticus and its association with an increased O-acetyl content of the cell wall. Nature. 1958 Jun 28;181(4626):1783–1784. doi: 10.1038/1811783a0. [DOI] [PubMed] [Google Scholar]
- Chin T., Burger M. M., Glaser L. Synthesis of teichoic acids. VI. The formation of multiple wall polymers in Bacillus subtilis W-23. Arch Biochem Biophys. 1966 Sep 26;116(1):358–367. doi: 10.1016/0003-9861(66)90042-7. [DOI] [PubMed] [Google Scholar]
- GHUYSEN J. M. Acetylhexosamine compounds enzymically released from Micrococcus lysodeikticus cell walls. II. Enzymic sensitivity of purified acetylhexosamine and acetylhexosamine-peptide complexes. Biochim Biophys Acta. 1960 Jun 3;40:473–480. doi: 10.1016/0006-3002(60)91388-3. [DOI] [PubMed] [Google Scholar]
- GHUYSEN J. M., SALTON M. R. Acetylhexosamine compounds enzymically released from Micrococcus lysodeikticus cell walls. I. Isolation and composition of acetylhexosamine and acetylhexosamine-peptide complexes. Biochim Biophys Acta. 1960 Jun 3;40:462–472. doi: 10.1016/0006-3002(60)91387-1. [DOI] [PubMed] [Google Scholar]
- GHUYSEN J. M. [Data on the structure of disaccharide-peptide complexes liberated from the wall of Micrococcus lysodeikticus by the action of beta(1-4)N-acetylhexosaminidases]. Biochim Biophys Acta. 1961 Mar 4;47:561–568. doi: 10.1016/0006-3002(61)90551-0. [DOI] [PubMed] [Google Scholar]
- Hughes R. C. The cell wall of Bacillus licheniformis N.C.T.C. 6346. Composition of the mucopeptide component. Biochem J. 1968 Jan;106(1):41–48. doi: 10.1042/bj1060041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes R. C. The cell wall of Bacillus licheniformis N.C.T.C. 6346: Biosynthesis of the teichuronic acid. Biochem J. 1966 Dec;101(3):692–697. doi: 10.1042/bj1010692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes R. C. The isolation of structural components present in the cell wall of Bacillus licheniformis N.C.T.C. 6346. Biochem J. 1965 Sep;96(3):700–709. doi: 10.1042/bj0960700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izaki K., Matsuhashi M., Strominger J. L. Glycopeptide transpeptidase and D-alanine carboxypeptidase: penicillin-sensitive enzymatic reactions. Proc Natl Acad Sci U S A. 1966 Mar;55(3):656–663. doi: 10.1073/pnas.55.3.656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JANCZURA E., PERKINS H. R., ROGERS H. J. Teichuronic acid: a mucopolysaccharide present in wall preparations from vegetative cells of Bacillus subtilis. Biochem J. 1961 Jul;80:82–93. doi: 10.1042/bj0800082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JUSIC D., ROY C., WATSON R. W. SEQUENCE STUDIES ON BACTERIAL CELL WALL PEPTIDES. Can J Biochem. 1964 Nov;42:1553–1559. doi: 10.1139/o64-166. [DOI] [PubMed] [Google Scholar]
- LEUTGEB W., WEIDEL W. OLIGO-MUCOPEPTIDE AUS DER STUETZMEMBRAN VON E. COLI. Z Naturforsch B. 1963 Dec;18:1065–1069. [PubMed] [Google Scholar]
- MANDELSTAM J., ROGERS H. J. The incorporation of amino acids into the cell-wall mucopeptide of staphylococci and the effect of antibiotics on the process. Biochem J. 1959 Aug;72:654–662. doi: 10.1042/bj0720654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MCCARTY M. The lysis of group A hemolytic streptococci by extracellular enzymes of Streptomyces albus. II. Nature of the cellular substrate attacked by the lytic enzymes. J Exp Med. 1952 Dec;96(6):569–580. doi: 10.1084/jem.96.6.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MITCHELL P., MOYLE J. The glycerol-phospho-protein complex envelope of Micrococcus pyogenes. J Gen Microbiol. 1951 Nov;5(5 Suppl):981–992. doi: 10.1099/00221287-5-5-981. [DOI] [PubMed] [Google Scholar]
- Martin H. H. Biochemistry of bacterial cell walls. Annu Rev Biochem. 1966;35:457–484. doi: 10.1146/annurev.bi.35.070166.002325. [DOI] [PubMed] [Google Scholar]
- Mirelman D., Sharon N. Isolation and characterization of two disaccharide-peptides from lysozyme digests of Micrococcus lysodeikticus cell walls. Biochem Biophys Res Commun. 1966 Jul 20;24(2):237–243. doi: 10.1016/0006-291x(66)90726-1. [DOI] [PubMed] [Google Scholar]
- PELZER H. MUCOPEPTIDHYDROLASEN IN ESCHERICHIA COLI B. I. NACHWEIS UND WIRKUNGSSPEZIFITAET. Z Naturforsch B. 1963 Nov;18:950–956. [PubMed] [Google Scholar]
- PERKINS H. R., ROGERS H. J. The products of the partial acid hydrolysis of the mucopeptide from cell walls of Micrococcus lysodeikticus. Biochem J. 1959 Aug;72:647–654. doi: 10.1042/bj0720647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PERKINS H. R. THE ACTION OF HOT FORMAMIDE ON BACTERIAL CELL WALLS. Biochem J. 1965 Jun;95:876–882. doi: 10.1042/bj0950876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PERKINS H. R. The structure of a disaccharide liberated by lysozyme from the cell walls of Micrococcus lysodeikticus. Biochem J. 1960 Jan;74:182–186. doi: 10.1042/bj0740182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PRIMOSIGH J., PELZER H., MAASS D., WEIDEL W. Chemical characterization of mucopeptides released from the E. coli B cell wall by enzymic action. Biochim Biophys Acta. 1961 Jan 1;46:68–80. doi: 10.1016/0006-3002(61)90647-3. [DOI] [PubMed] [Google Scholar]
- Rogers H. J. Separable polymers in bacterial cell walls. Br Med Bull. 1966 May;22(2):185–189. doi: 10.1093/oxfordjournals.bmb.a070464. [DOI] [PubMed] [Google Scholar]
- SALTON M. R. J., HORNE R. W. Studies of the bacterial cell wall. II. Methods of preparation and some properties of cell walls. Biochim Biophys Acta. 1951 Jul;7(2):177–197. doi: 10.1016/0006-3002(51)90017-0. [DOI] [PubMed] [Google Scholar]
- SALTON M. R., GHUYSEN J. M. Acetylhexosamine compounds enzymically released from Micrococcus lysodeikticus cell walls. III. The structure of DI- and tetra-saccharides released from cell walls by lysozyme and Streptomyces F1 enzyme. Biochim Biophys Acta. 1960 Dec 4;45:355–363. doi: 10.1016/0006-3002(60)91458-x. [DOI] [PubMed] [Google Scholar]
- SALTON M. R., GHUYSEN J. M. The structure of di- and tetrasaccharides released from cell walls by lysozyme and Streptomyces F 1 enzyme and the beta(1 to 4) N-acetylhexos-aminidase activity of these enzymes. Biochim Biophys Acta. 1959 Dec;36:552–554. doi: 10.1016/0006-3002(59)90205-7. [DOI] [PubMed] [Google Scholar]
- SALTON M. R. Studies of the bacterial cell wall. V. The action of lysozyme on cell walls of some lysozyme-sensitive bacteria. Biochim Biophys Acta. 1956 Dec;22(3):495–506. doi: 10.1016/0006-3002(56)90060-9. [DOI] [PubMed] [Google Scholar]
- SALTON M. R. Studies of the bacterial cell wall. VIII. Reaction of walls with hydrazine and with fluorodinitrobenzene. Biochim Biophys Acta. 1961 Sep 16;52:329–342. doi: 10.1016/0006-3002(61)90682-5. [DOI] [PubMed] [Google Scholar]
- SALTON M. R. The properties of lysozyme and its action on microorganisms. Bacteriol Rev. 1957 Jun;21(2):82–100. doi: 10.1128/br.21.2.82-100.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHOCHER A. J., JUSIC D., WATSON R. W. Hydrazinolysis of purified mucopeptide from the wall of Aerobacter cloacae. Biochem Biophys Res Commun. 1961 Oct 23;6:16–19. doi: 10.1016/0006-291x(61)90176-0. [DOI] [PubMed] [Google Scholar]
- Sharon N., Osawa T., Flowers H. M., Jeanloz R. W. Isolation and study of the chemical structure of a disaccharide from Micrococcus lysodeikticus cell walls. J Biol Chem. 1966 Jan 10;241(1):223–230. [PubMed] [Google Scholar]
- TAKEBE I. EXTENT OF CROSS LINKAGE IN THE MUREIN SACCULUS OF ESCHERICHIA COLI B CELL WALL. Biochim Biophys Acta. 1965 Mar 1;101:124–126. doi: 10.1016/0926-6534(65)90038-2. [DOI] [PubMed] [Google Scholar]
- WEIDEL W., PELZER H. BAGSHAPED MACROMOLECULES--A NEW OUTLOOK ON BACTERIAL CELL WALLS. Adv Enzymol Relat Areas Mol Biol. 1964;26:193–232. doi: 10.1002/9780470122716.ch5. [DOI] [PubMed] [Google Scholar]
- YPHANSTIS D. A. Rapid determination of molecular weights of peptides and preteins. Ann N Y Acad Sci. 1960 Aug 31;88:586–601. doi: 10.1111/j.1749-6632.1960.tb20055.x. [DOI] [PubMed] [Google Scholar]
- Young F. E. Variation in the chemical composition of the cell walls of Bacillus subtilis during growth in different media. Nature. 1965 Jul 3;207(992):104–105. doi: 10.1038/207104b0. [DOI] [PubMed] [Google Scholar]


