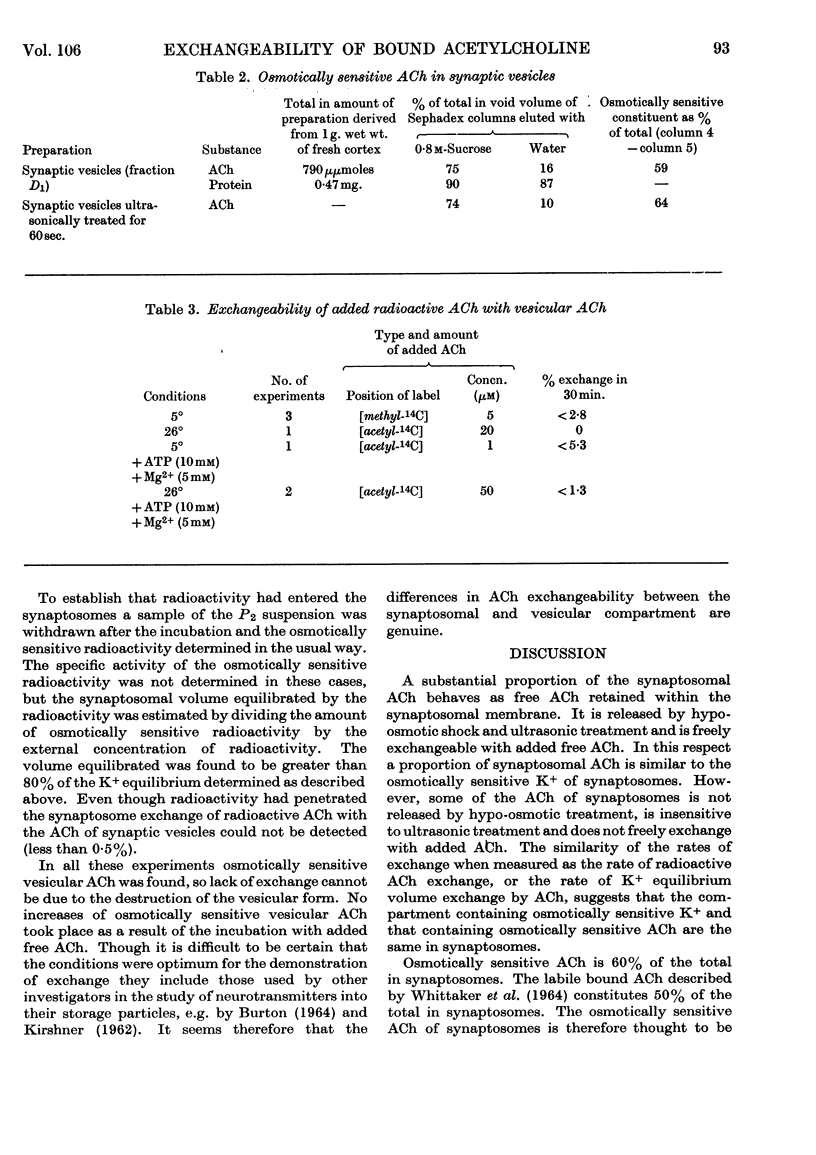
Abstract
1. The exchangeability with added radioactive acetylcholine of the acetylcholine in isolated presynaptic nerve terminals (synaptosomes) and isolated synaptic vesicles was studied by a Sephadex-column method. 2. A substantial proportion of the synaptosomal acetylcholine is exchangeable with added radioactive acetylcholine. It is liberated by hypo-osmotic shock and ultrasonic treatment, and behaves as though it occupies the cytoplasmic compartment of synaptosomes. 3. Methods of isolating vesicles from hypo-osmotically ruptured synaptosomes in optimum yield are discussed. 4. The acetylcholine of synaptic vesicles isolated on a sucrose density gradient is released by hypo-osmotic conditions, suggesting that it is enclosed by a semi-permeable membrane; however, it is not easily released by ultrasonic treatment. 5. Added radioactive acetylcholine does not exchange with vesicular acetylcholine under a variety of different conditions. These include addition of ATP and Mg2+, and pre-loading of the synaptosome with radioactive acetylcholine before hypo-osmotic rupture. This failure to exchange is discussed in terms of the possible storage mechanism of vesicular acetylcholine.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BURTON R. M. GANGLIOSIDES AND ACETYLCHOLINE OF THE CENTRAL NERVOUS SYSTEM. 3. THE BINDING OF RADIOACTIVE ACETYLCHOLINE BY SUBCELLULAR PARTICLES OF THE BRAIN. Int J Neuropharmacol. 1964 Apr;3:13–21. doi: 10.1016/0028-3908(64)90040-1. [DOI] [PubMed] [Google Scholar]
- Colburn R. W., Maas J. W. Adenosine triphosphate--metal--norepinephrine ternary complexes and catecholamine binding. Nature. 1965 Oct 2;208(5005):37–41. doi: 10.1038/208037a0. [DOI] [PubMed] [Google Scholar]
- DE ROBERTIS E., RODRIGUEZ DE LORES ARNAIZ G., SALGANICOFF L., PELLEGRINO DE IRALDI A., ZIEHER L. M. Isolation of synaptic vesicles and structural organization of the acetycholine system within brain nerve endings. J Neurochem. 1963 Apr;10:225–235. doi: 10.1111/j.1471-4159.1963.tb05038.x. [DOI] [PubMed] [Google Scholar]
- KIRSHNER N. Uptake of catecholamines by a particulate fraction of the adrenal medulla. J Biol Chem. 1962 Jul;237:2311–2317. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Marchbanks R. M. Compartmentation of acetylcholine in synaptosomes. Biochem Pharmacol. 1967 May;16(5):921–923. doi: 10.1016/0006-2952(67)90068-8. [DOI] [PubMed] [Google Scholar]
- Marchbanks R. M. The osmotically sensitive potassium and sodium compartments of synaptosomes. Biochem J. 1967 Jul;104(1):148–157. doi: 10.1042/bj1040148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SALGANICOFF L., DEROBERTIS E. SUBCELLULAR DISTRIBUTION OF THE ENZYMES OF THE GLUTAMIC ACID, GLUTAMINE AND GAMMA-AMINOBUTYRIC ACID CYCLES IN RAT BRAIN. J Neurochem. 1965 Apr;12:287–309. doi: 10.1111/j.1471-4159.1965.tb06766.x. [DOI] [PubMed] [Google Scholar]
- Sattin A. The synthesis and storage of acetylcholine in the striatum. J Neurochem. 1966 Jun;13(6):515–524. doi: 10.1111/j.1471-4159.1966.tb09866.x. [DOI] [PubMed] [Google Scholar]
- WHITTAKER V. P., SHERIDAN M. N. THE MORPHOLOGY AND ACETYLCHOLINE CONTENT OF ISOLATED CEREBRAL CORTICAL SYNAPTIC VESICLES. J Neurochem. 1965 May;12:363–372. doi: 10.1111/j.1471-4159.1965.tb04237.x. [DOI] [PubMed] [Google Scholar]
- WHITTAKER V. P. The isolation and characterization of acetylcholine-containing particles from brain. Biochem J. 1959 Aug;72:694–706. doi: 10.1042/bj0720694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittaker V. P., Michaelson I. A., Kirkland R. J. The separation of synaptic vesicles from nerve-ending particles ('synaptosomes'). Biochem J. 1964 Feb;90(2):293–303. doi: 10.1042/bj0900293. [DOI] [PMC free article] [PubMed] [Google Scholar]