Abstract
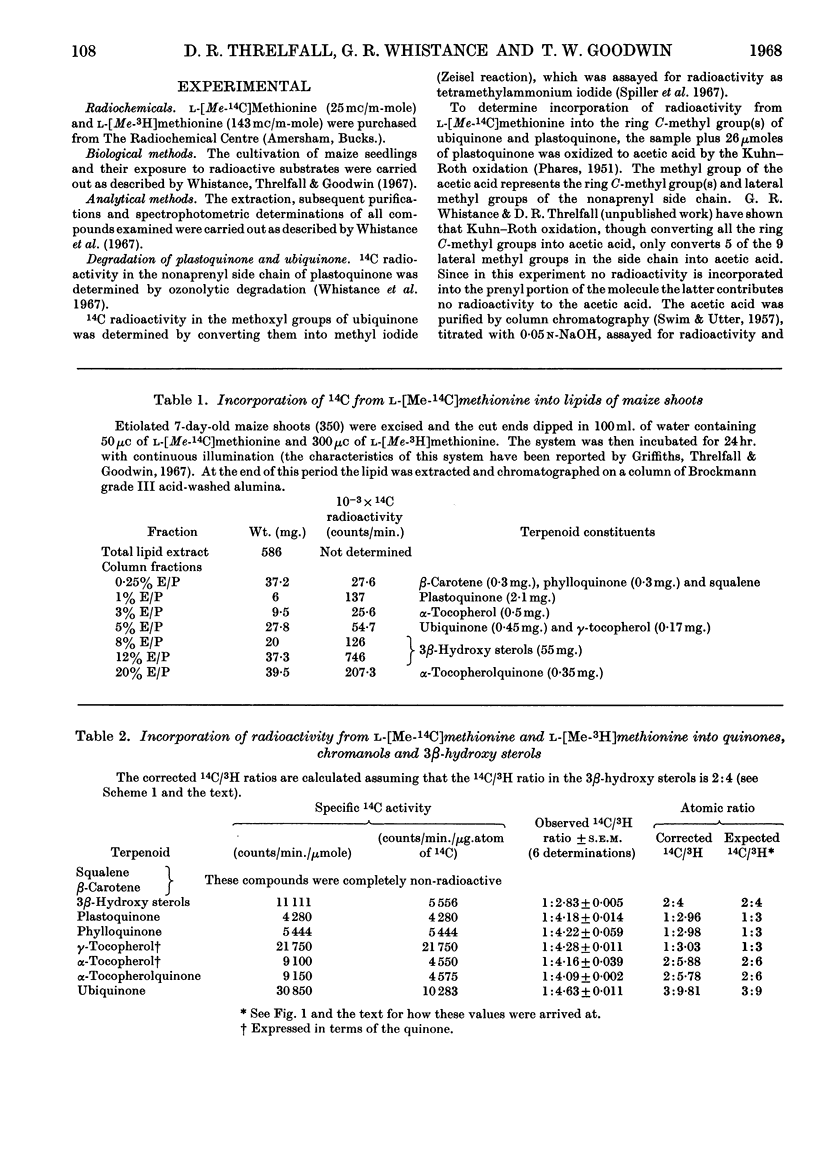
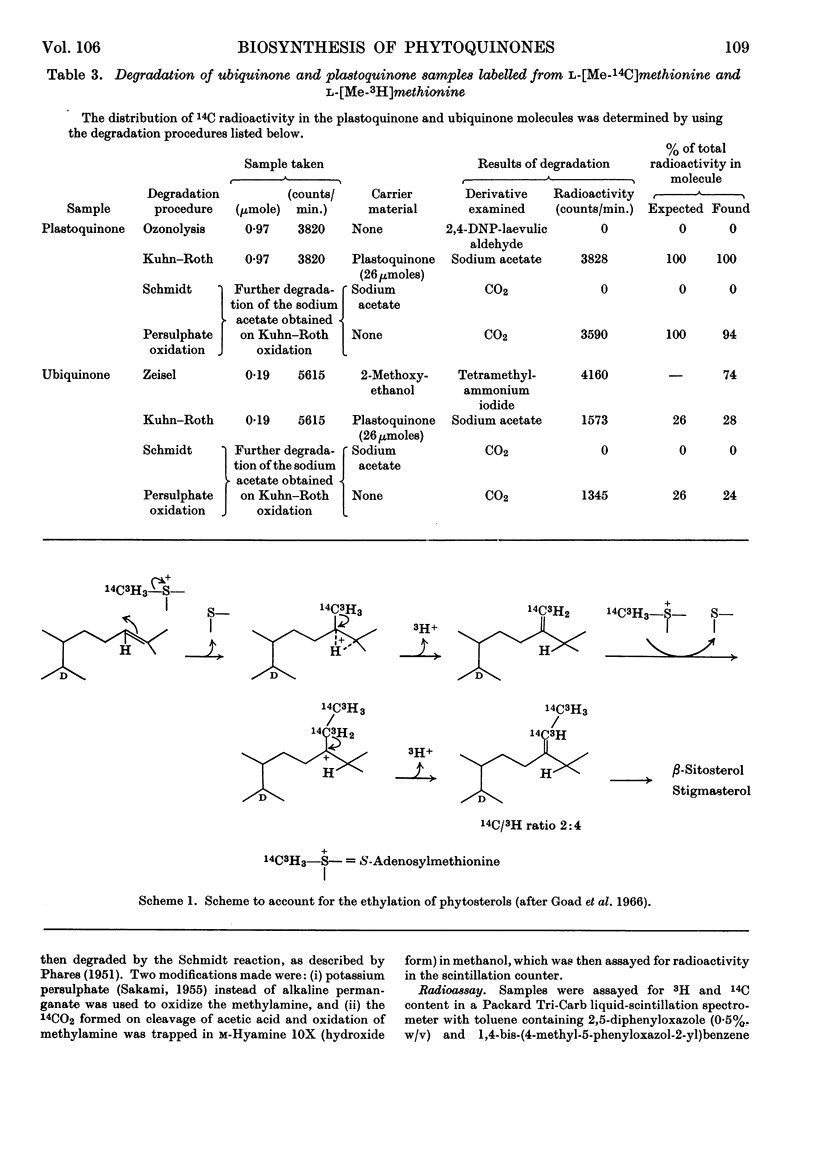
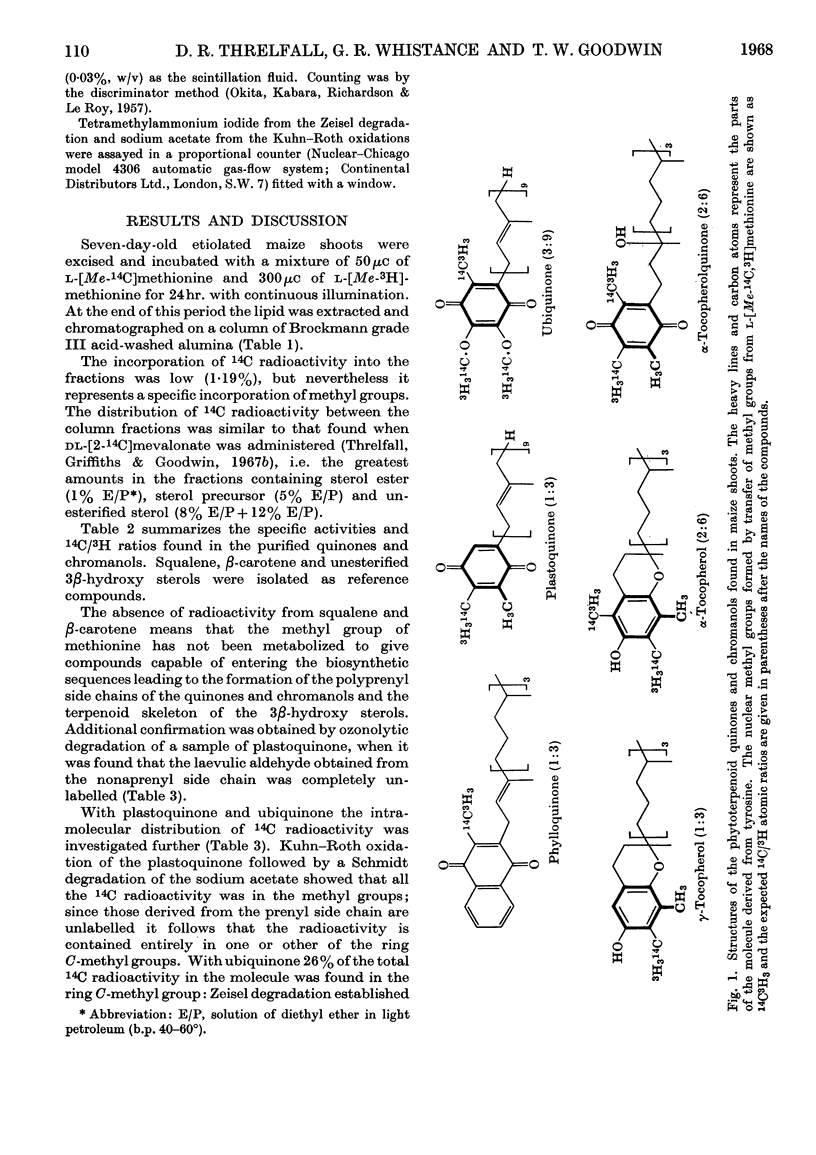
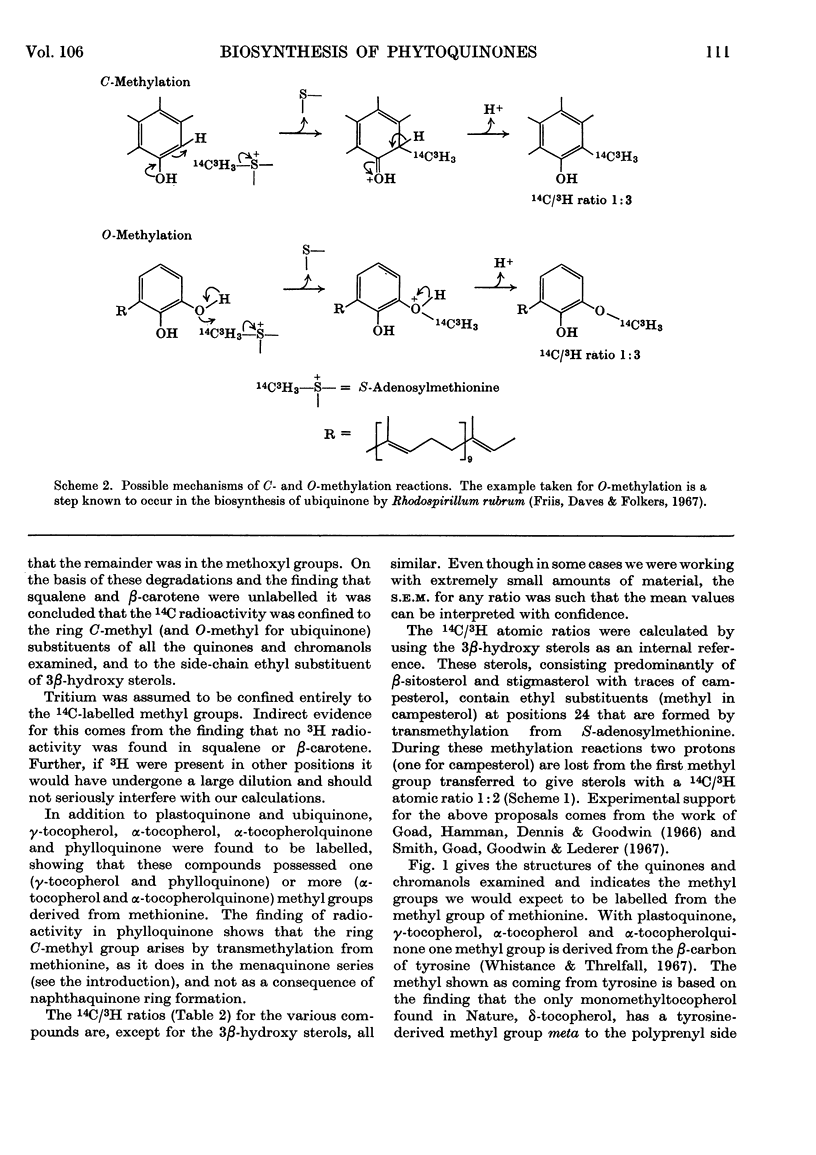
1. Radioactivity from l-[Me-14C,3H]methionine is incorporated into phylloquinone, plastoquinone, γ-tocopherol, α-tocopherol, α-tocopherolquinone and ubiquinone in maize shoots. 2. Comparative studies with other terpenoids (squalene and β-carotene) and chemical degradation of selected quinones (ubiquinone and plastoquinone) established that all the radioactivity is confined to nuclear methyl substituents. 3. In ubiquinone 76% of the radioactivity is in the methoxyl groups and 24% in the ring C-methyl group. 4. Taking the phytosterols as an internal reference and accepting the atomic ratio of 14C/3H transferred from l-[Me-14C,3H]methionine to the supernumerary group at C24 to be 1:2 the ratio of all the quinones and chromanols examined approached 1:3. After allowing for the fact that for plastoquinone, γ-tocopherol, α-tocopherol and α-tocopherolquinone one nuclear methyl group is formed from the β-carbon of tyrosine, these results show that one nuclear C-methyl group for phylloquinone, plastoquinone and γ-tocopherol, two nuclear methyl groups for α-tocopherol and α-tocopherolquinone and one nuclear methyl and two methoxyl groups for ubiquinone are formed by the transfer of intact methyl groups from methionine. 5. From a comparison of the incorporation of 14C radioactivity into these compounds it would appear that the methylation reactions involved in phylloquinone and plastoquinone biosynthesis take place in the chloroplast, whereas those involved with ubiquinone biosynthesis occur else-where within the cell.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Griffiths W. T., Threlfall D. R., Goodwin T. W. Nature, intracellular distribution and formation of terpenoid quinones in maize and barley shoots. Biochem J. 1967 May;103(2):589–600. doi: 10.1042/bj1030589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guérin M., Azerad R., Lederer E. Sur l'origine biogénétique du méthyle en position 2 de la vitamine K2 de Mycobacterium phlei. Bull Soc Chim Biol (Paris) 1965;47(11):2105–2114. [PubMed] [Google Scholar]
- PHARES E. F. Degradation of labeled propionic and acetic acids. Arch Biochem Biophys. 1951 Sep;33(2):173–178. doi: 10.1016/0003-9861(51)90094-x. [DOI] [PubMed] [Google Scholar]
- Threlfall D. R., Griffiths W. T., Goodwin T. W. Biosynthesis of the prenyl side chains of plastoquinone and related compounds in maize and barley shoots. Biochem J. 1967 Jun;103(3):831–851. doi: 10.1042/bj1030831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whistance G. R., Threlfall D. R. Biosynthesis of phytoquinones: an outline of the biosynthetic sequences involved in terpenoid quinone and chromanol formation by higher plants. Biochem Biophys Res Commun. 1967 Aug 7;28(3):295–301. doi: 10.1016/0006-291x(67)90308-7. [DOI] [PubMed] [Google Scholar]
- Whistance G. R., Threlfall D. R., Goodwin T. W. Observations on the biosynthesis of phytoterpenoid quinone and chromanol nuclei. Biochem J. 1967 Oct;105(1):145–154. doi: 10.1042/bj1050145. [DOI] [PMC free article] [PubMed] [Google Scholar]


