Abstract
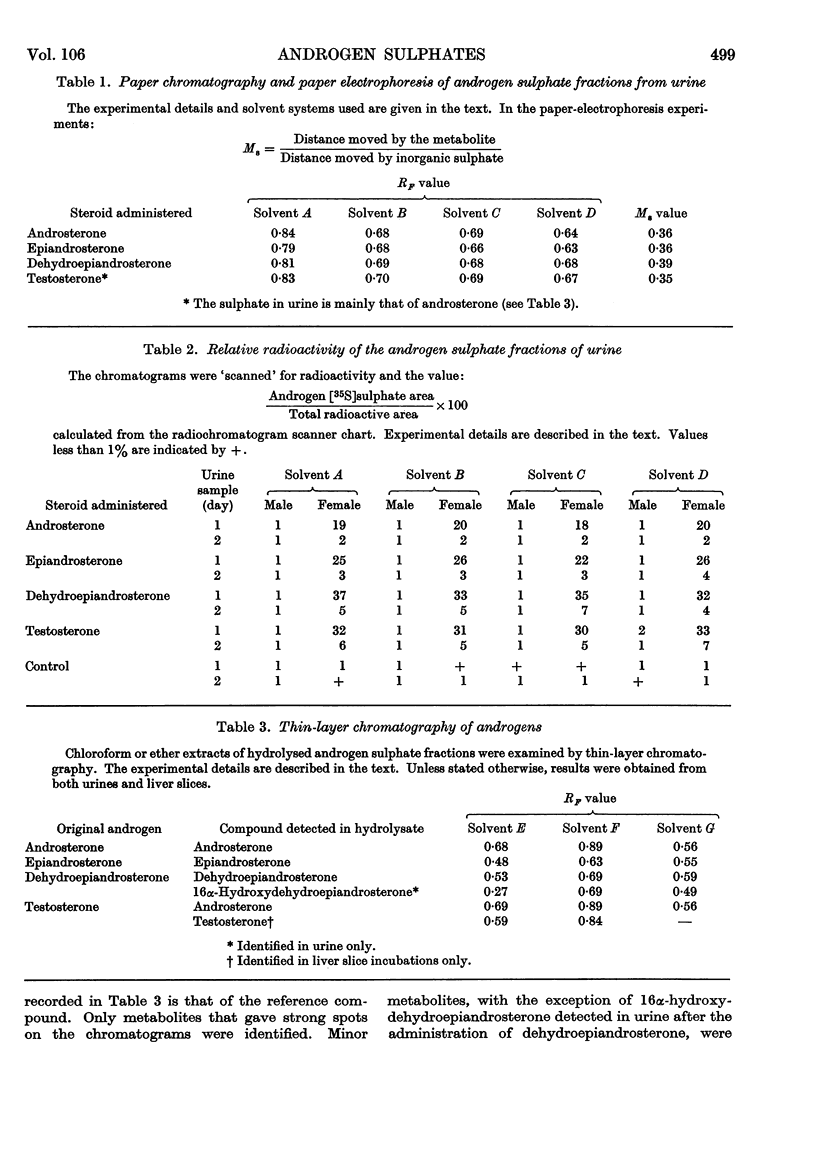
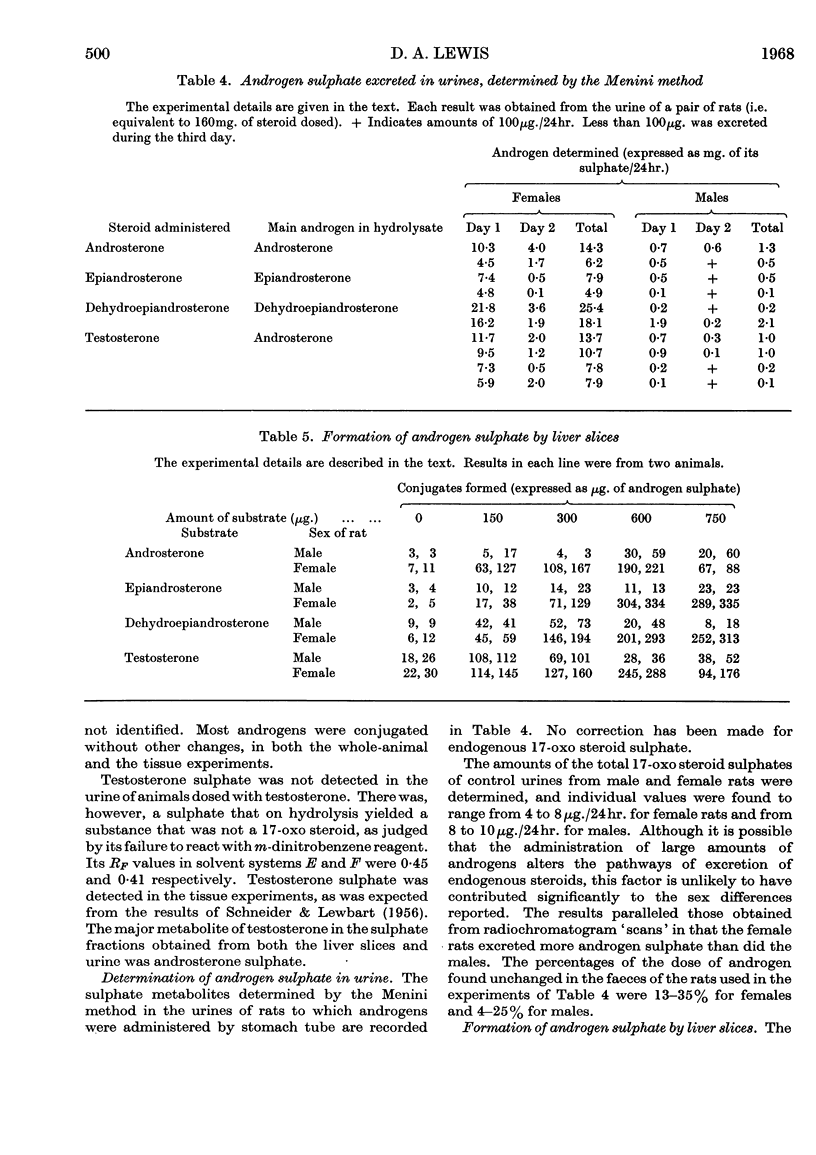
1. After large doses of androsterone, epiandrosterone, dehydroepiandrosterone and testosterone, female rats excreted more of the dose conjugated with sulphuric acid than did the males. 2. Androgens were also incubated with liver slices from male and female rats. Slices from females conjugated androgens with sulphuric acid to a greater extent than did slices from males. 3. The amount of unchanged androgen present in the faeces of orally dosed animals was 4–35% of the dose.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson F. B., Cunningham W. L., Manners D. J. Studies on carbohydrate-metabolizing enzymes. 10. Barley beta-glucosidases. Biochem J. 1964 Jan;90(1):30–35. doi: 10.1042/bj0900030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BAULIEU E. E., CORPECHOT C., DRAY F., EMILIOZZI R., LEBEAU M. C., MAUVAIS JARVIS P., ROBEL P. AN ADRENAL-SECRETED "ANDROGEN": DEHYDROISOANDROSTERONE SULFATE. ITS METABOLISM AND A TENTATIVE GENERALIZATION ON THE METABOLISM OF OTHER STEROID CONJUGATES IN MAN. Recent Prog Horm Res. 1965;21:411–500. [PubMed] [Google Scholar]
- CALVIN H. I., LIEBERMAN S. EVIDENCE THAT STEROID SULFATES SERVE AS BIOSYNTHETIC INTERMEDIATES. II. IN VITRO CONVERSION OF PREGNENOLONE-3H SULFATE-35S TO 17ALPHA-HYDROXYPREGNENOLONE-3H SULFATE-35S. Biochemistry. 1964 Feb;3:259–264. doi: 10.1021/bi00890a020. [DOI] [PubMed] [Google Scholar]
- CALVIN H. I., VANDEWIELE R. L., LIEBERMAN S. EVIDENCE THAT STEROID SULFATES SERVE AS BIOSYNTHETIC INTERMEDIATES: IN VIVO CONVERSION OF PREGNENOLONE-SULFATE-S35 TO DEHYDROISOANDROSTERONE SULFATE-S35. Biochemistry. 1963 Jul-Aug;2:648–653. doi: 10.1021/bi00904a005. [DOI] [PubMed] [Google Scholar]
- COLAS A. The 16alpha-hydroxylation of dehydroepiandrosterone (3 beta-hydroxyandrost-5-en-17-one) by rat-liver slices. Biochem J. 1962 Mar;82:390–394. doi: 10.1042/bj0820390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CORKER C. S., NORYMBERSKI J. K., THOW R. Some aspects of the Zimmermann reaction. Biochem J. 1962 Jun;83:583–588. doi: 10.1042/bj0830583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DICKERSON J. W. The effect of development on the composition of a long bone of the pig, rat and fowl. Biochem J. 1962 Jan;82:47–55. doi: 10.1042/bj0820047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FORCHIELLI E., BROWN-GRANT K., DORFMAN R. I. Steroid delta 4-hydrogenases of rat liver. Proc Soc Exp Biol Med. 1958 Dec;99(3):594–596. doi: 10.3181/00379727-99-24430. [DOI] [PubMed] [Google Scholar]
- HAGEN A. A., TROOP R. C. Influence of age, sex and adrenocortical status on hepatic reduction of cortisone in vitro. Endocrinology. 1960 Aug;67:194–203. doi: 10.1210/endo-67-2-194. [DOI] [PubMed] [Google Scholar]
- KELLIE A. E., WADE A. P. The analysis of urinary 17-oxo steroids by gradient elution. Biochem J. 1957 Jun;66(2):196–206. doi: 10.1042/bj0660196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEWBART M. L., SCHNEIDER J. J. Enzymatic synthesis of steroid sulfates. J Biol Chem. 1956 Oct;222(2):787–794. [PubMed] [Google Scholar]
- Menini E. The indirect analysis of 17-oxosteroid glucosiduronates and sulphates in human urine. Biochem J. 1966 Jun;99(3):747–759. doi: 10.1042/bj0990747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RAO L. G., TAYLOR W. THE METABOLISM OF PROGESTERONE BY ANIMAL TISSUES IN VITRO. SEX AND SPECIES DIFFERENCES IN CONJUGATE FORMATION DURING THE METABOLISM OF (4-14C)PROGESTERONE BY LIVER HOMOGENATES. Biochem J. 1965 Jul;96:172–180. doi: 10.1042/bj0960172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROY A. B. Comparative studies on the liver sulphatases. Biochem J. 1958 Mar;68(3):519–528. doi: 10.1042/bj0680519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROY A. B. The enzymic synthesis of steroid sulphates. Biochem J. 1956 Jun;63(2):294–300. doi: 10.1042/bj0630294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RUBIN B. L., STRECKER H. J. Further studies on the sex difference in 3 beta-hydroxysteroid dehydrogenase activity of rat livers. Endocrinology. 1961 Aug;69:257–267. doi: 10.1210/endo-69-2-257. [DOI] [PubMed] [Google Scholar]
- RUBIN B. L. Sex differences in orientation of reduction products of 3-keto-C19 steroids by rat liver homogenates. J Biol Chem. 1957 Aug;227(2):917–927. [PubMed] [Google Scholar]
- Raggatt P. R., Whitehouse M. W. Substrate and inhibitor specificity of the cholesterol oxidase in bovine adrenal cortex. Biochem J. 1966 Dec;101(3):819–830. doi: 10.1042/bj1010819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YATES F. E., HERBST A. L., URQUHART J. Sex difference in rate of ring A reduction of delta 4-3-keto-steroids in vitro by rat liver. Endocrinology. 1958 Dec;63(6):887–902. doi: 10.1210/endo-63-6-887. [DOI] [PubMed] [Google Scholar]


