Abstract
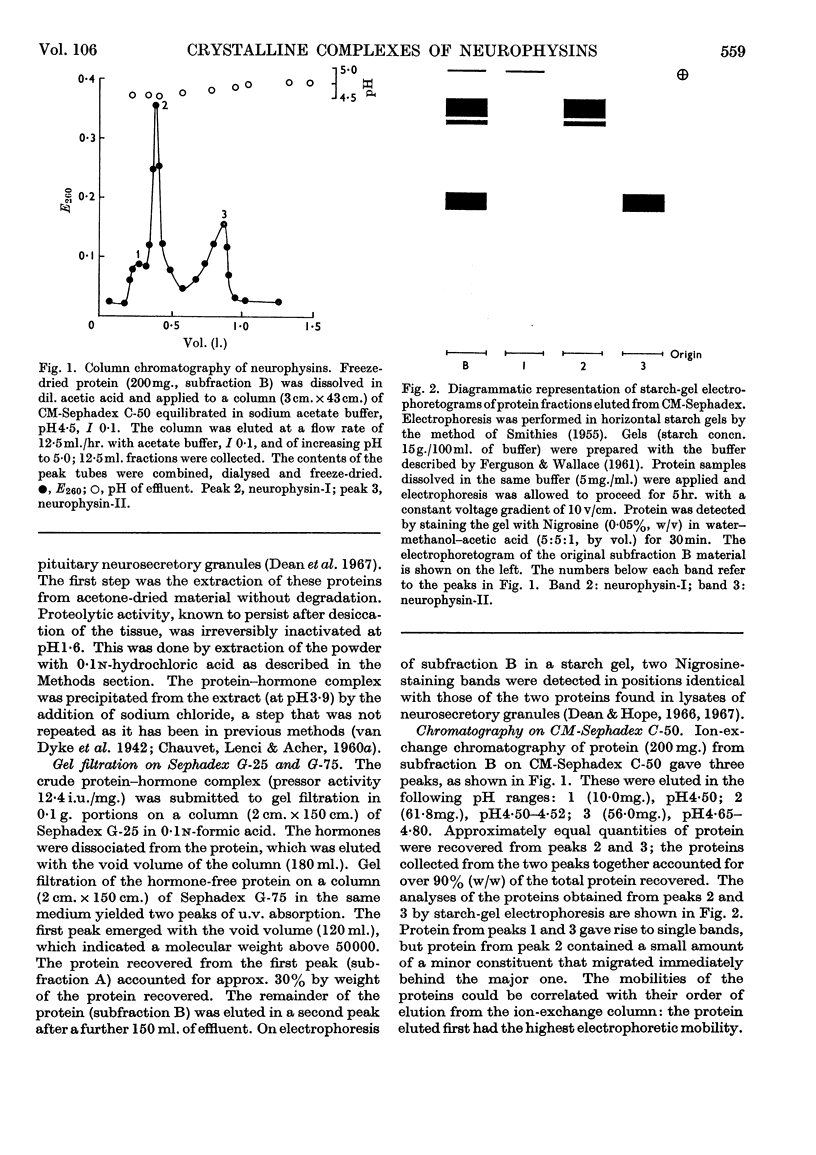
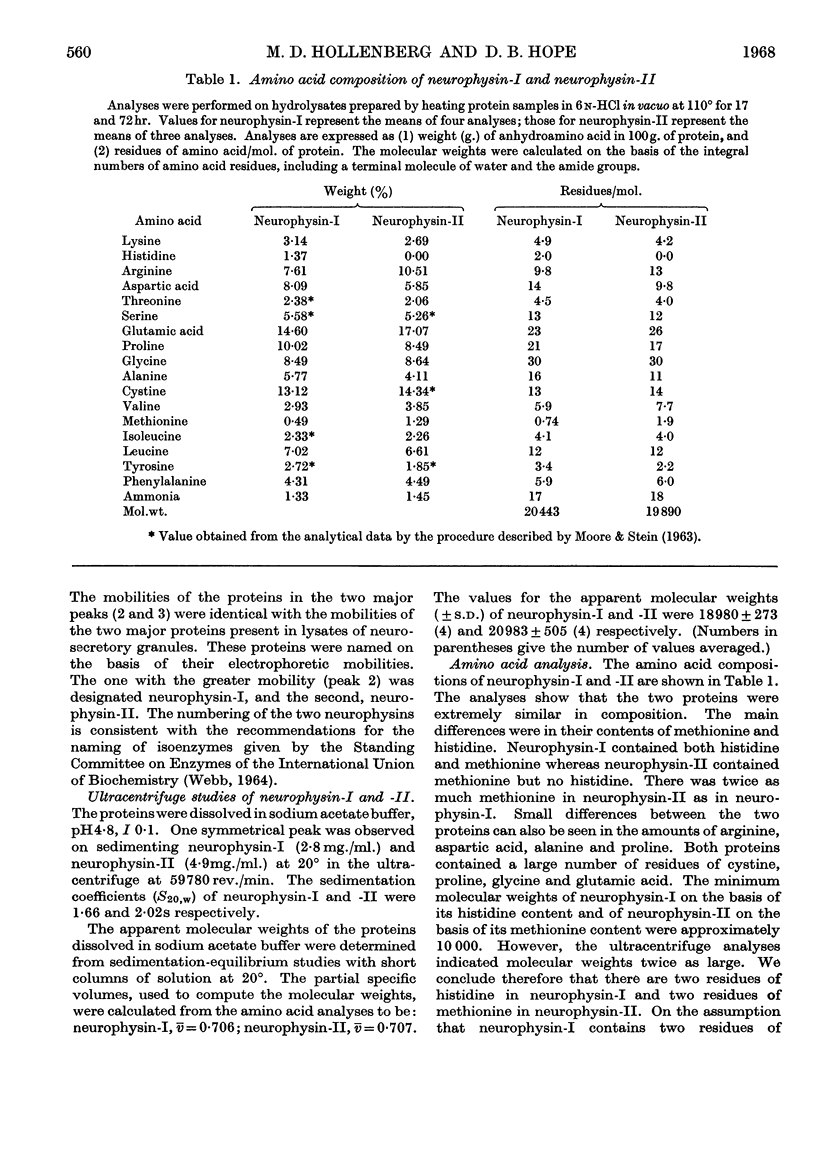
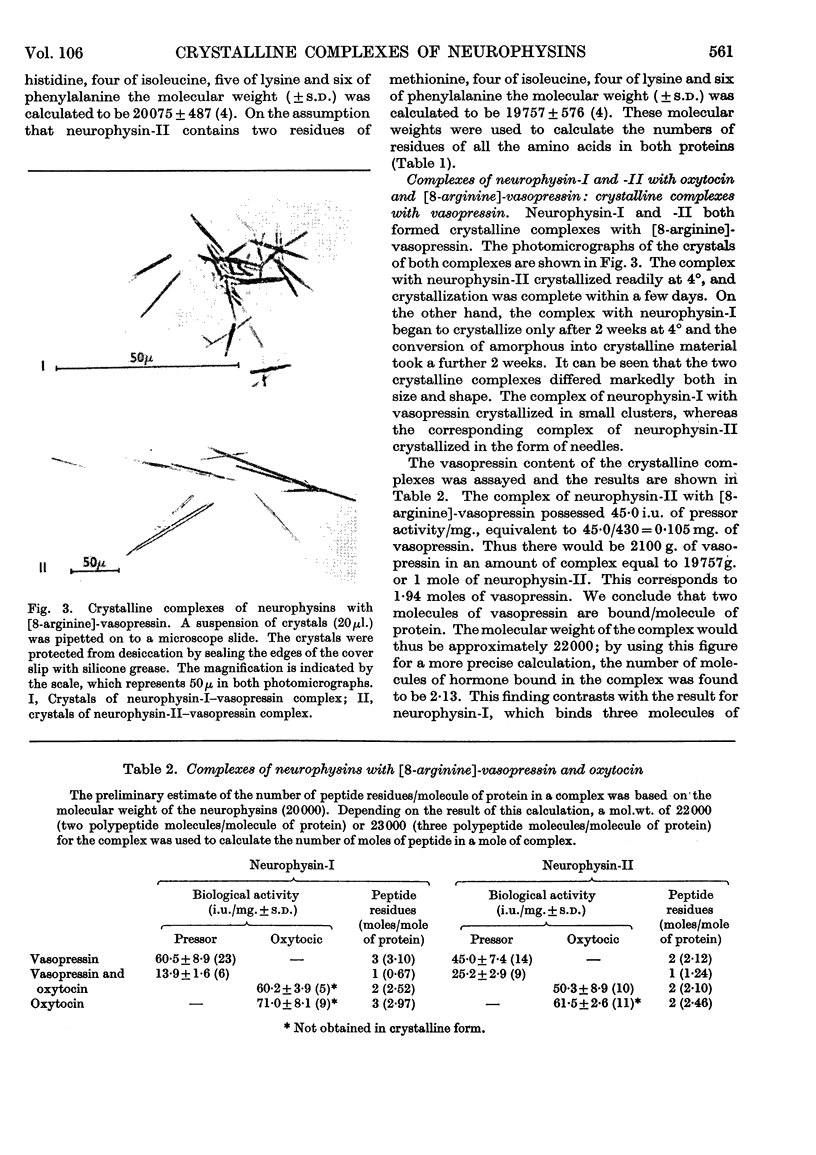
1. The native hormone-binding proteins, neurophysin-I and -II, have been isolated from acetone-desiccated bovine pituitary posterior lobes. 2. Neurophysin-I and -II are present in approximately equal quantities in the tissue and are localized in the neurosecretory granules. 3. The apparent molecular weight, determined by equilibrium sedimentation of neurophysin-I, was 19000 and that of neurophysin-II was 21000; their sedimentation coefficients, S20,w, were 1·66 and 2·02s respectively. 4. Neurophysin-I and -II are similar in amino acid composition. Neurophysin-II was distinguished from neurophysin-I by the absence of histidine. 5. The proteins form complexes with oxytocin as well as with vasopressin. Complexes of both proteins with [8-arginine]-vasopressin have been crystallized. 6. Bioassay of the pressor and oxytocic activities of the crystals shows that neurophysin-I binds three molecules of either vasopressin or oxytocin whereas neurophysin-II binds only two molecules of each hormone per molecule of protein. Complexes containing two molecules of oxytocin and one molecule of [8-arginine]-vasopressin per molecule of protein are formed by neurophysin-I and -II; both proteins appear to possess three polypeptide-binding sites/molecule.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ACHER R., CHAUVET J., LENCI M. T., MOREL F., MAETZ J. [Presence of a vasotocin in the neurohypophysis of the frog (Rana esculenta L.)]. Biochim Biophys Acta. 1960 Aug 12;42:379–380. doi: 10.1016/0006-3002(60)90814-3. [DOI] [PubMed] [Google Scholar]
- ACHER R., CHAUVET J., OLIVRY G. Sur l'existence éventuelle d'une hormone unique neurohypophysaire. I. Relations entre l'ocytocine, la vasopressine et la protéine de Van Dyke extraites de la neurohypophyse du boeuf. Biochim Biophys Acta. 1956 Dec;22(3):421–427. doi: 10.1016/0006-3002(56)90050-6. [DOI] [PubMed] [Google Scholar]
- BANGHAM D. R., MUSSETT M. V. Third international standard for posterior pituitary; re-named third international standard for oxytocic, vasopressor and antidiuretic substances in 1956. Bull World Health Organ. 1958;19(2):325–340. [PMC free article] [PubMed] [Google Scholar]
- Breslow E., Abrash L. The binding of oxytocin and oxytocin analogues by purified bovine neurophysins. Proc Natl Acad Sci U S A. 1966 Aug;56(2):640–646. doi: 10.1073/pnas.56.2.640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHAN W. Y., O'CONNELL M., POMEROY S. R. Effects of the estrous cycle on the sensitivity of rat uterus to oxytocin and desamino-oxytocin. Endocrinology. 1963 Feb;72:279–282. doi: 10.1210/endo-72-2-279. [DOI] [PubMed] [Google Scholar]
- CHAUVET J., LENCI M. T., ACHER R. [Oxytocin and vasopressin of sheep: reconstitution of an active hormonal complex]. Biochim Biophys Acta. 1960 Feb 26;38:266–272. doi: 10.1016/0006-3002(60)91241-5. [DOI] [PubMed] [Google Scholar]
- CHAUVET J., LENCI M. T., ACHER R. [Presence of 2 vasopressins in the neurohypophysis of the chick]. Biochim Biophys Acta. 1960 Mar 11;38:571–573. doi: 10.1016/0006-3002(60)91301-9. [DOI] [PubMed] [Google Scholar]
- CRESTFIELD A. M., MOORE S., STEIN W. H. The preparation and enzymatic hydrolysis of reduced and S-carboxymethylated proteins. J Biol Chem. 1963 Feb;238:622–627. [PubMed] [Google Scholar]
- DEKANSKI J. The quantitative assay of vasopressin. Br J Pharmacol Chemother. 1952 Dec;7(4):567–572. doi: 10.1111/j.1476-5381.1952.tb00723.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean C. R., Hope D. B. The isolation of neurophysin-I and-II from bovine pituitary neurosecretory granules separated on a large scale from other subcellular organelles. Demonstration of slow equilibration of neurosecretory granules during centrifugation in a sucrose density gradient. Biochem J. 1968 Jan;106(2):565–573. doi: 10.1042/bj1060565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean C. R., Hope D. B. The isolation of purified neurosecretory granules from bovine pituitary posterior lobes. Comparison of granule protein constituents with those of neurophysin. Biochem J. 1967 Sep;104(3):1082–1088. doi: 10.1042/bj1041082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FERGUSON K. A., WALLACE A. L. Starch-gel electrophoresis of anterior pituitary hormones. Nature. 1961 May 13;190:629–630. doi: 10.1038/190629a0. [DOI] [PubMed] [Google Scholar]
- Frankland B. T., Hollenberg M. D., Hope D. B., Schacter B. A. Dissociation of oxytocin and vasopressin from their carrier protein by chromatography on sephadex G-25. Br J Pharmacol Chemother. 1966 Feb;26(2):502–510. doi: 10.1111/j.1476-5381.1966.tb01931.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GAITAN E., COBO E., MIZRACHI M. EVIDENCE FOR THE DIFFERENTIAL SECRETION OF OXYTOCIN AND VASOPRESSIN IN MAN. J Clin Invest. 1964 Dec;43:2310–2322. doi: 10.1172/JCI105105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GINSBURG M., IRELAND M. THE PREPARATION OF BOVINE NEUROPHYSIN AND THE ESTIMATION OF ITS MAXIMUM CAPACITY TO BIND OXYTOCIN AND ARGININE VASOPRESSIN. J Endocrinol. 1965 May;32:187–198. doi: 10.1677/joe.0.0320187. [DOI] [PubMed] [Google Scholar]
- Hollenberg M. D., Hope D. B. Fractionation of neurophysin by molecular-sieve and ion-exchange chromatography. Biochem J. 1967 Jul;104(1):122–127. doi: 10.1042/bj1040122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollenberg M. D., Hope D. B. The composition of crystalline complexes of neurophysin-M with [8-arginine]-vasopressin and oxytocin. Biochem J. 1967 Dec;105(3):921–926. doi: 10.1042/bj1050921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KELLY C. D., LAYNE S. Bacteria found in the air over Canada and the American Arctic. Can J Microbiol. 1957 Apr;3(3):447–455. doi: 10.1139/m57-047. [DOI] [PubMed] [Google Scholar]
- MUNSICK R. A. Effect of magnesium ion on the response of the rat uterus to neurohypophysial hormones and analogues. Endocrinology. 1960 Mar;6:451–457. doi: 10.1210/endo-66-3-451. [DOI] [PubMed] [Google Scholar]
- SAWYER W. H. Neurophypophysial hormones. Pharmacol Rev. 1961 Jun;13:225–277. [PubMed] [Google Scholar]
- SMITHIES O. Zone electrophoresis in starch gels: group variations in the serum proteins of normal human adults. Biochem J. 1955 Dec;61(4):629–641. doi: 10.1042/bj0610629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WADDELL W. J. A simple ultraviolet spectrophotometric method for the determination of protein. J Lab Clin Med. 1956 Aug;48(2):311–314. [PubMed] [Google Scholar]
- YPHANSTIS D. A. Rapid determination of molecular weights of peptides and preteins. Ann N Y Acad Sci. 1960 Aug 31;88:586–601. doi: 10.1111/j.1749-6632.1960.tb20055.x. [DOI] [PubMed] [Google Scholar]



