Abstract
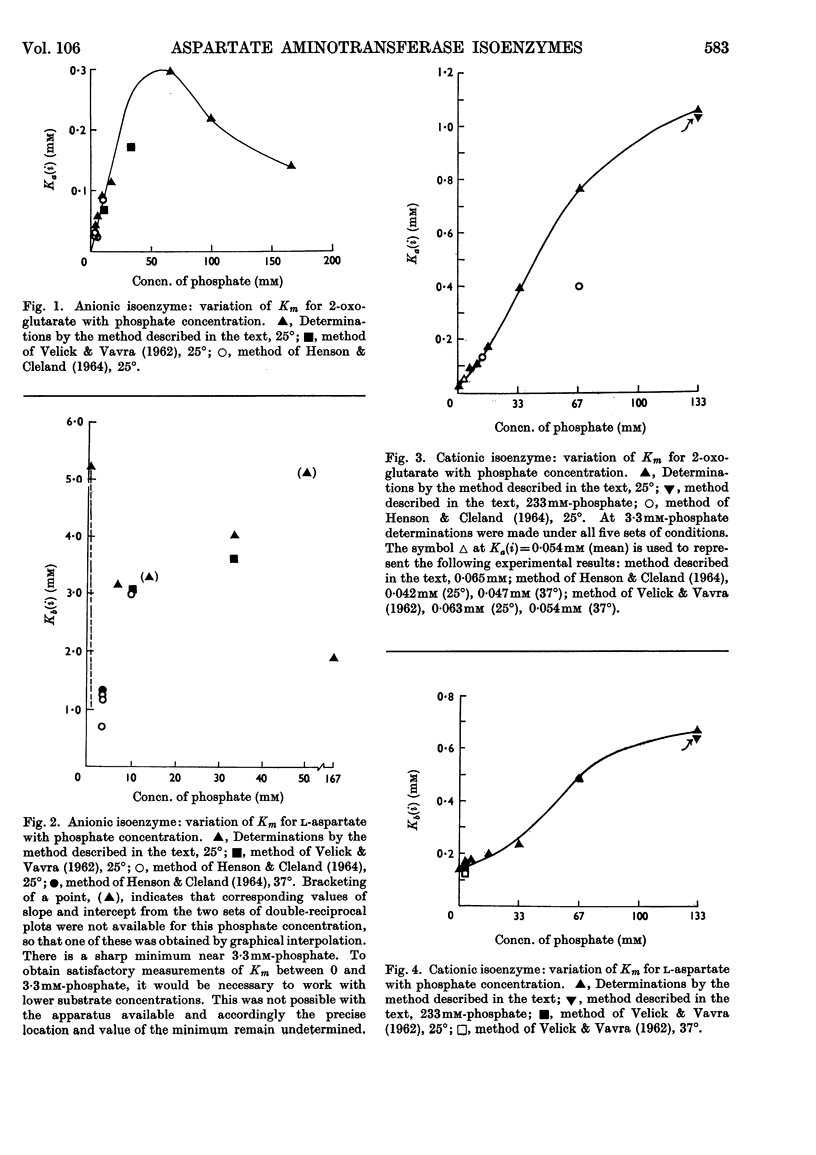
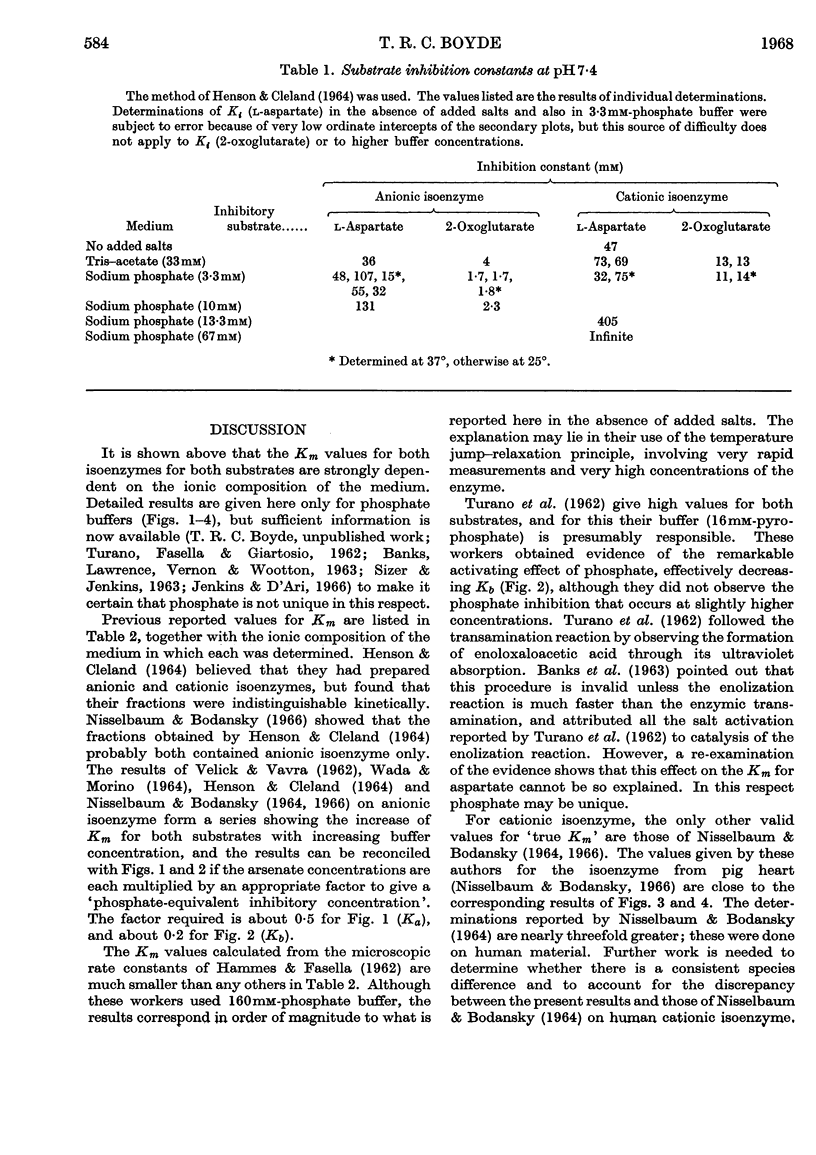
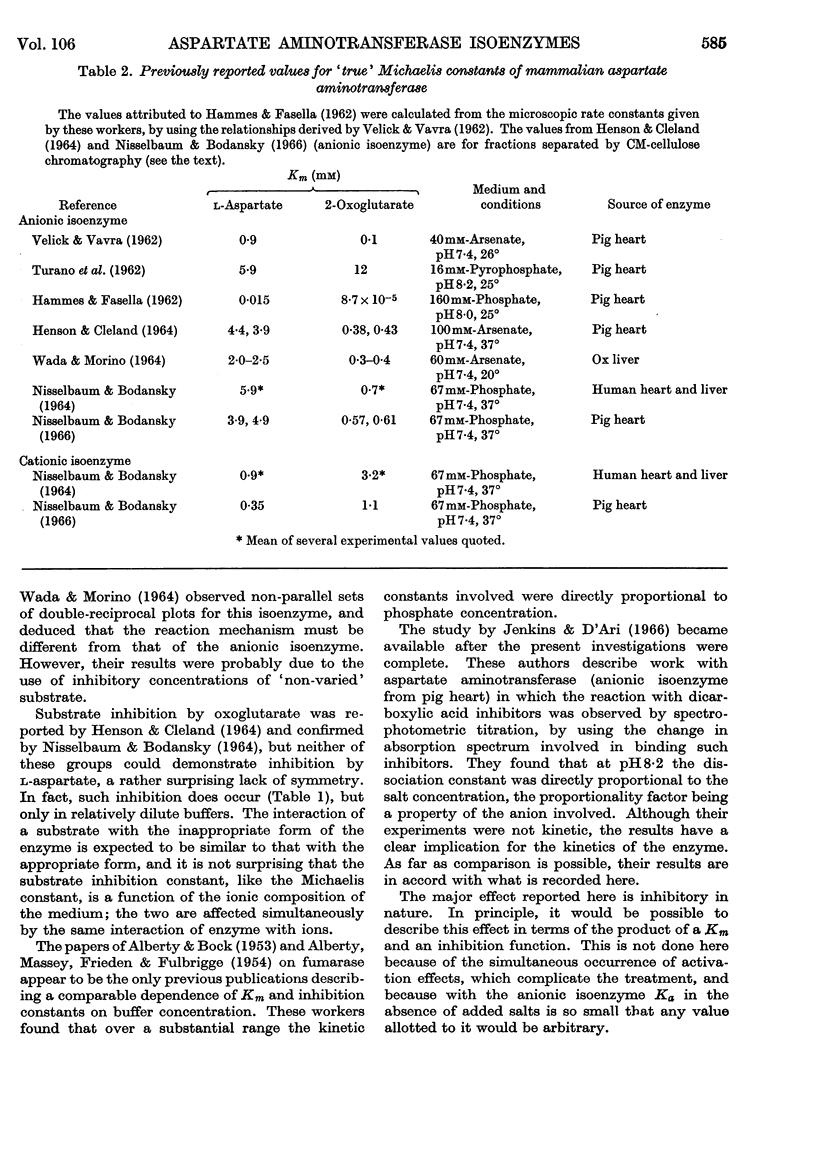
1. The Michaelis constants for both isoenzymes for both substrates depend strongly on ionic concentration, being approximately proportional to phosphate concentration over considerable ranges. This is probably an effect of anions only. 2. In the absence of added salt, Km (2-oxoglutarate) (anionic isoenzyme) is so small as to be indeterminate. 3. Km (l-aspartate) (anionic isoenzyme) passes through a sharp minimum at about 3·3mm-phosphate. It is not clear whether this is a specific effect of phosphate. 4. Both substrates are inhibitory at sufficiently low ionic concentrations. 5. A modified graphical procedure is described for the derivation of the kinetic constants.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alberty R. A., Bock R. M. Alteration of the Kinetic Properties of an Enzyme by the Binding of Buffer, Inhibitor, or Substrate. Proc Natl Acad Sci U S A. 1953 Sep;39(9):895–900. doi: 10.1073/pnas.39.9.895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BORST P., PEETERS E. M. The intracellular localization of glutamate-oxaloacetate transaminases in heart. Biochim Biophys Acta. 1961 Nov 25;54:188–189. doi: 10.1016/0006-3002(61)90953-2. [DOI] [PubMed] [Google Scholar]
- BOYD J. W. The intracellular distribution, latency and electrophoretic mobility of L-glutamate-oxaloacetate transaminase from rat liver. Biochem J. 1961 Nov;81:434–441. doi: 10.1042/bj0810434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FLEISHER G. A., POTTER C. S., WAKIM K. G. Separation of 2 glutamic-oxalacetic transaminases by paper electrophoresis. Proc Soc Exp Biol Med. 1960 Jan;103:229–231. doi: 10.3181/00379727-103-25469. [DOI] [PubMed] [Google Scholar]
- HENSON C. P., CLELAND W. W. KINETIC STUDIES OF GLUTAMIC OXALOACETIC TRANSAMINASE ISOZYMES. Biochemistry. 1964 Mar;3:338–345. doi: 10.1021/bi00891a007. [DOI] [PubMed] [Google Scholar]
- Jenkins W. T., D'Ari L. Glutamic-aspartic transaminase. X. Mechanism and order of formation of the enzyme-substrate carboxylate bonds. J Biol Chem. 1966 Dec 10;241(23):5667–5674. [PubMed] [Google Scholar]
- KARMEN A. A note on the spectrometric assay of glutamic-oxalacetic transaminase in human blood serum. J Clin Invest. 1955 Jan;34(1):131–133. [PubMed] [Google Scholar]
- MOORE B. W., LEE R. H. Chromatography of rat liver soluble proteins and localization of enzyme activities. J Biol Chem. 1960 May;235:1359–1364. [PubMed] [Google Scholar]
- NISSELBAUM J. S., BODANSKY O. IMMUNOCHEMICAL AND KINETIC PROPERTIES OF ANIONIC AND CATIONIC GLUTAMIC-OXALOACETIC TRANSAMINASES SEPARATED FROM HUMAN HEART AND HUMAN LIVER. J Biol Chem. 1964 Dec;239:4232–4236. [PubMed] [Google Scholar]
- Nisselbaum J. S., Bodansky O. Kinetics and electrophoretic properties of the isozymes of aspartate aminotransferase from pig heart. J Biol Chem. 1966 Jun 10;241(11):2661–2664. [PubMed] [Google Scholar]
- TURANO C., FASELLA P., GIARTOSIO A. On the effect of small ions on the activity of glutamic-aspartic transaminase. Biochim Biophys Acta. 1962 Apr 9;58:255–261. doi: 10.1016/0006-3002(62)91007-7. [DOI] [PubMed] [Google Scholar]
- VELICK S. F., VAVRA J. A kinetic and equilibrium analysis of the glutamic oxaloacetate transaminase mechanism. J Biol Chem. 1962 Jul;237:2109–2122. [PubMed] [Google Scholar]
- WADA H., MORINO Y. COMPARATIVE STUDIES ON GLUTAMIC-OXALACETIC TRANSAMINASES FROM THE MITOCHONDRIAL AND SOLUBLE FRACTIONS OF MAMMALIAN TISSUES. Vitam Horm. 1964;22:411–444. doi: 10.1016/s0083-6729(08)60346-5. [DOI] [PubMed] [Google Scholar]


