Abstract
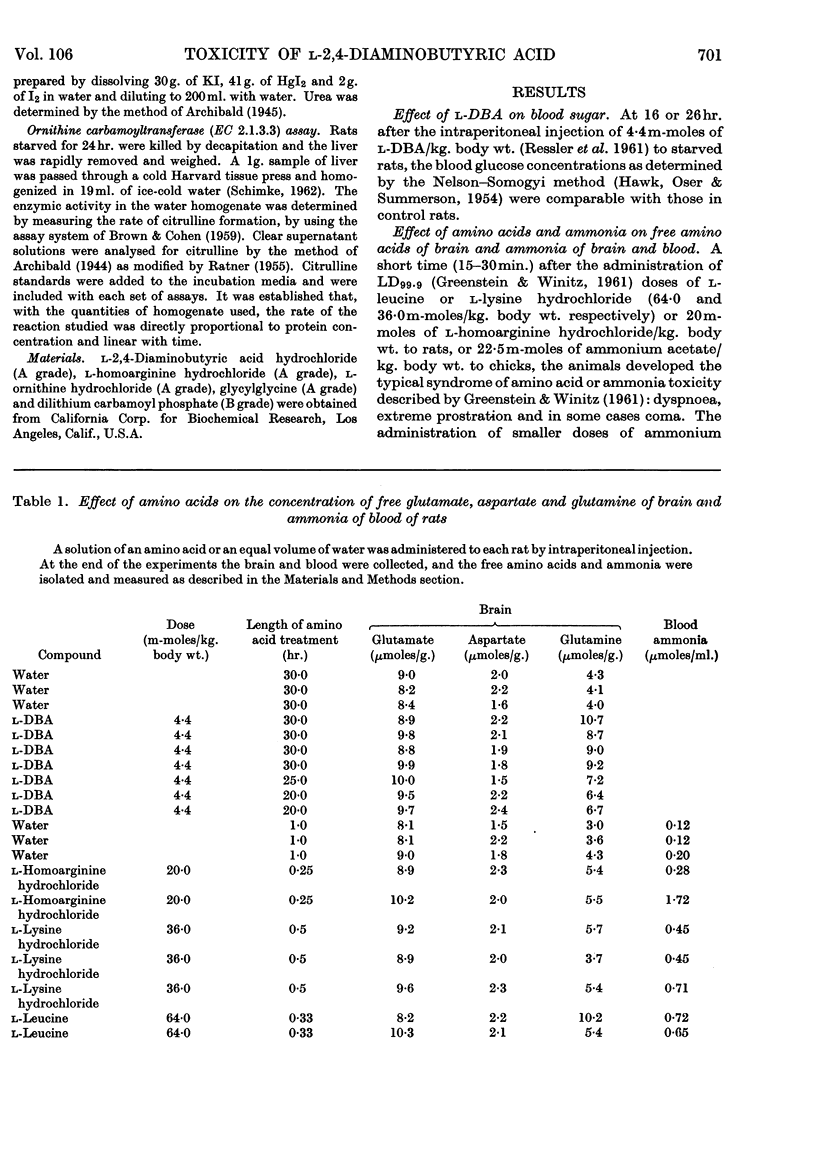
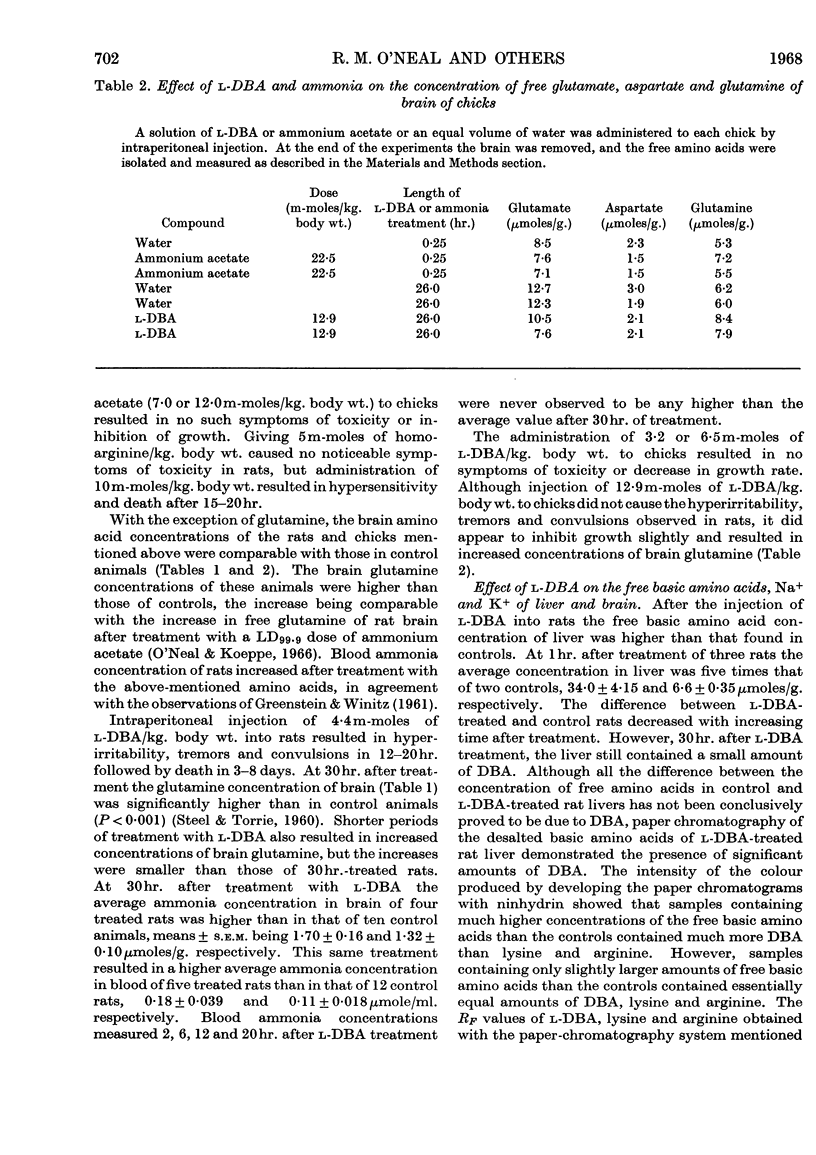
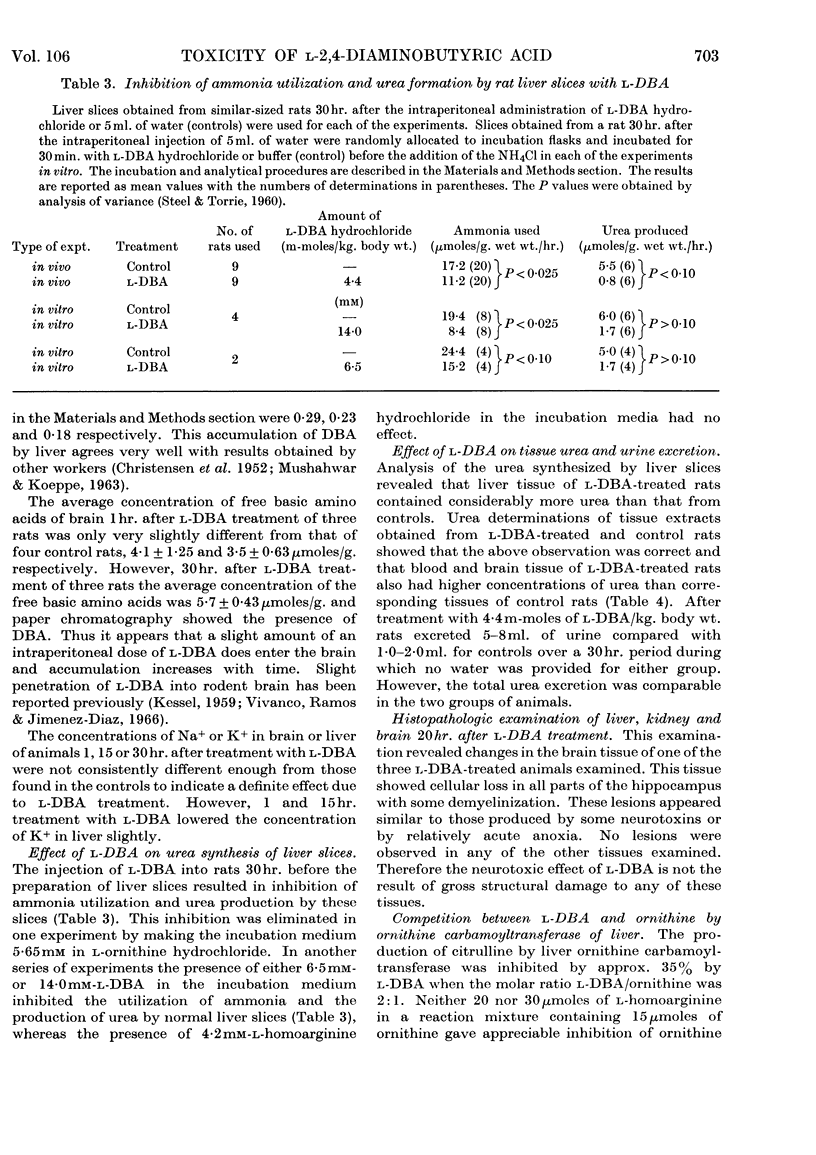
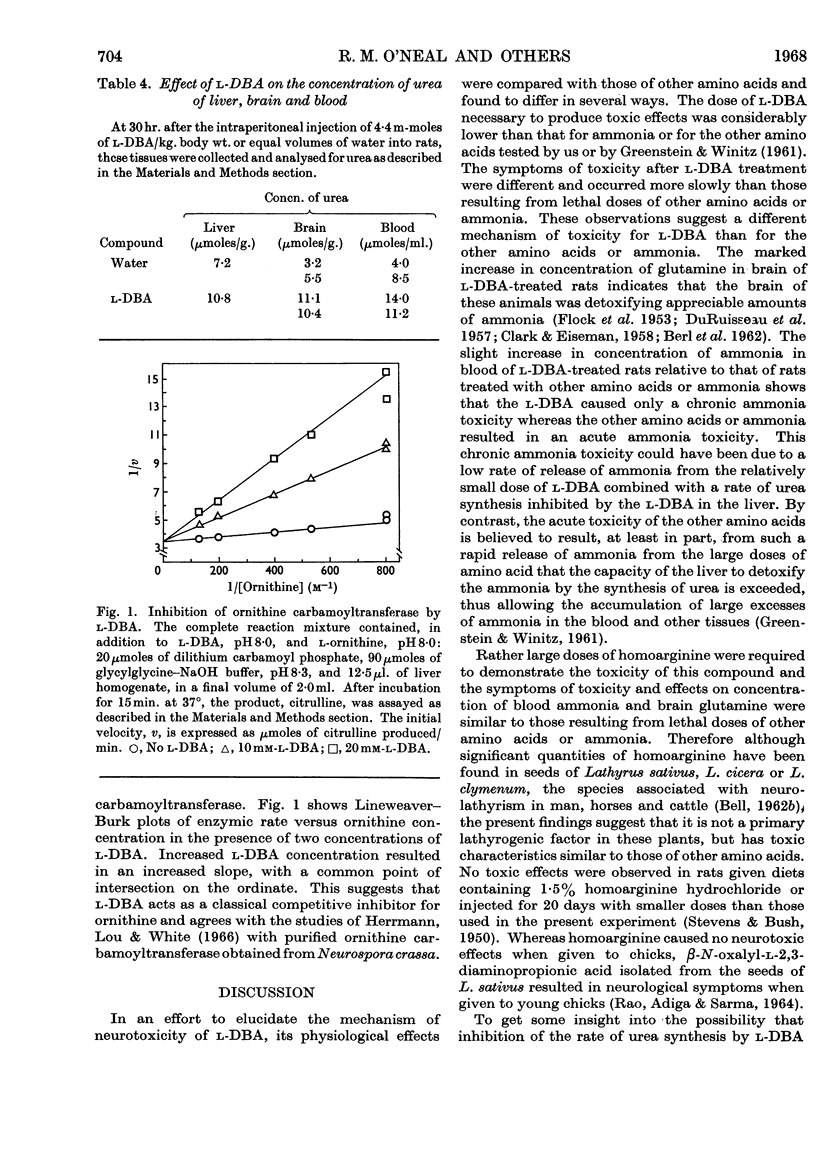
The neurolathyrogen l-2,4-diaminobutyric acid is concentrated by liver, and liver damage can yield neurotoxicity; thus the neurotoxicity caused by this compound may be due to liver damage followed by secondary brain damage. 1. The intraperitoneal administration of toxic doses of l-2,4-diaminobutyric acid to rats resulted in hyperirritability, tremors and convulsions in 12–20hr. and increased the concentration of ammonia of blood and brain slightly and the concentration of glutamine of brain two- to three-fold. By contrast, toxic doses of l-homoarginine, l-lysine, l-leucine and ammonium acetate caused dyspnoea, extreme prostration, and in some cases coma in 15–30min., and increased the concentration of ammonia of blood significantly and the concentration of glutamine of brain slightly. These results indicate that l-2,4-diaminobutyric acid caused a chronic ammonia toxicity, whereas the other amino acids and ammonium acetate resulted in an acute ammonia toxicity. 2. Liver slices from l-2,4-diaminobutyric acid-treated animals and normal liver slices preincubated with l-2,4-diaminobutyric acid utilized ammonia and formed urea at a lower rate than control slices from normal rats. 3. l-2,4-Diaminobutyric acid inhibited competitively ornithine carbamoyltransferase of rat liver homogenates, thus demonstrating that this reaction is a primary site of toxicity for this neurolathyrogen. Although we were unable to show marked elevations of blood ammonia concentration after treatment with l-2,4-diaminobutyric acid, these results are interpreted to mean that ammonia utilization (urea synthesis) in liver is inhibited by l-2,4-diaminobutyric acid and that at least part of the neurotoxicity is due to a prolonged slight increase in body ammonia concentration.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BELL E. A. Associations of ninhydrin-reacting compounds in the seeds of 49 species of Lathyrus. Biochem J. 1962 May;83:225–229. doi: 10.1042/bj0830225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BELL E. A. alpha,gamma-Diaminobutyric acid in seeds of twelve species of Lathyrus and identification of a new natural amino-acid, L-homoarginine, in seeds of other species toxic to man and domestic animals. Nature. 1962 Mar 17;193:1078–1079. doi: 10.1038/1931078b0. [DOI] [PubMed] [Google Scholar]
- BERL S., TAKAGAKI G., CLARKE D. D., WAELSCH H. Metabolic compartments in vivo. Ammonia and glutamic acid metabolism in brain and liver. J Biol Chem. 1962 Aug;237:2562–2569. [PubMed] [Google Scholar]
- BIRNBAUM S. M., GREENSTEIN J. P., GULLINO P., OTEY M. C., WINITZ M. Studies on the metabolism of amino acids and related compounds in vivo. III. Prevention of ammonia toxicity by arginine and related compounds. Arch Biochem Biophys. 1956 Oct;64(2):342–354. doi: 10.1016/0003-9861(56)90278-8. [DOI] [PubMed] [Google Scholar]
- BROWN G. W., Jr, COHEN P. P. Comparative biochemistry of urea synthesis. I. Methods for the quantitative assay of urea cycle enzymes in liver. J Biol Chem. 1959 Jul;234(7):1769–1774. [PubMed] [Google Scholar]
- Bell E. A., Tirimanna A. S. Associations of amino acids and related compounds in the seeds of forty-seven species of Vicia: their taxonomic and nutritional significance. Biochem J. 1965 Oct;97(1):104–111. doi: 10.1042/bj0970104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHRISTENSEN H. N., RIGGS T. R., FISCHER H., PALATINE I. M. Intense concentration of alpha, gamma-diaminobutyric acid by cells. J Biol Chem. 1952 Sep;198(1):17–22. [PubMed] [Google Scholar]
- CHRISTENSEN H. N., RIGGS T. R. Structural evidences for chelation and Schiff's base formation in amino acid transfer into cells. J Biol Chem. 1956 May;220(1):265–278. [PubMed] [Google Scholar]
- CLARK G. M., EISEMAN B. Studies in ammonia metabolism. IV. Biochemical changes in brain tissue of dogs during ammoniainduced coma. N Engl J Med. 1958 Jul 24;259(4):178–180. doi: 10.1056/NEJM195807242590406. [DOI] [PubMed] [Google Scholar]
- FLOCK E. V., BLOCK M. A., GRINDLAY J. H., MANN F. C., BOLLMAN J. L. Changes in free amino acids of brain and muscle after total hepatectomy. J Biol Chem. 1953 Feb;200(2):529–536. [PubMed] [Google Scholar]
- GRANT D. R. Reagent stability in Rosen's ninhydrin method of analysis for amino acids. Anal Biochem. 1963 Jul;6:109–110. doi: 10.1016/0003-2697(63)90013-7. [DOI] [PubMed] [Google Scholar]
- MOORE S., STEIN W. H. Procedures for the chromatographic determination of amino acids on four per cent cross-linked sulfonated polystyrene resins. J Biol Chem. 1954 Dec;211(2):893–906. [PubMed] [Google Scholar]
- MUSHAHWAR I. K., KOEPPE R. E. Concerning the metabolism of D- and L-alpha, gamma-diaminobutyric acid-2-C14 in rats. J Biol Chem. 1963 Jul;238:2460–2463. [PubMed] [Google Scholar]
- NATHAN D. G., RODKEY F. L. A colorimetric procedure for the determination of blood ammonia. J Lab Clin Med. 1957 May;49(5):779–785. [PubMed] [Google Scholar]
- O'Neal R. M., Koeppe R. E. Precursors in vivo of glutamate, aspartate and their derivatives of rat brain. J Neurochem. 1966 Sep;13(9):835–847. doi: 10.1111/j.1471-4159.1966.tb05879.x. [DOI] [PubMed] [Google Scholar]
- PAL P. R., CHRISTENSEN H. N. Interrelationships in the cellular uptake of amino acids and metals. J Biol Chem. 1959 Mar;234(3):613–617. [PubMed] [Google Scholar]
- PERKINS H. R., CUMMINS C. S. CHEMICAL STRUCTURE OF BACTERIAL CELL WALLS. ORNITHINE AND 2,4-DIAMINOBUTYRIC ACID AS COMPONENTS OF THE CELL WALLS OF PLANT PATHOGENIC CORYNEBACTERIA. Nature. 1964 Mar 14;201:1105–1107. doi: 10.1038/2011105a0. [DOI] [PubMed] [Google Scholar]
- RAO S. L., ADIGA P. R., SARMA P. S. THE ISOLATION AND CHARACTERIZATION OF BETA-N-OXALYL-L-ALPHA,BETA-DIAMINOPROPIONIC ACID: A NEUROTOXIN FROM THE SEEDS OF LATHYRUS SATIVUS. Biochemistry. 1964 Mar;3:432–436. doi: 10.1021/bi00891a022. [DOI] [PubMed] [Google Scholar]
- RESSLER C., REDSTONE P. A., ERENBERG R. H. Isolation and identification of a neuroactive factor from Lathyrus latifolius. Science. 1961 Jul 21;134(3473):188–190. doi: 10.1126/science.134.3473.188. [DOI] [PubMed] [Google Scholar]
- RIGGS T. R., COYNE B. A., CHRISTENSEN H. N. Amino acid concentration by a free cell neoplasm; structural influences. J Biol Chem. 1954 Jul;209(1):395–411. [PubMed] [Google Scholar]
- SCHIMKE R. T. Adaptive characteristics of urea cycle enzymes in the rat. J Biol Chem. 1962 Feb;237:459–468. [PubMed] [Google Scholar]
- SELIGSON D., SELIGSON H. A microdiffusion method for the determination of nitrogen liberated as ammonia. J Lab Clin Med. 1951 Aug;38(2):324–330. [PubMed] [Google Scholar]
- TEWS J. K., CARTER S. H., ROA P. D., STONE W. E. FREE AMINO ACIDS AND RELATED COMPOUNDS IN DOG BRAIN: POST-MORTEM AND ANOXIC CHANGES, EFFECTS OF AMMONIUM CHLORIDE INFUSION, AND LEVELS DURING SEIZURES INDUCED BY PICROTOXIN AND BY PENTYLENETETRAZOL. J Neurochem. 1963 Sep;10:641–653. doi: 10.1111/j.1471-4159.1963.tb08936.x. [DOI] [PubMed] [Google Scholar]
- Vivanco F., Ramos F., Jimenez-Diaz C. Determination of gamma-aminobutyric acid and other free amino acids in whole brains of rats poisoned with beta, beta'-iminodipropionitrile and alpha, gamma-diaminobutyric acid with, or without, administration of thyroxine. J Neurochem. 1966 Dec;13(12):1461–1467. doi: 10.1111/j.1471-4159.1966.tb04307.x. [DOI] [PubMed] [Google Scholar]


