Abstract
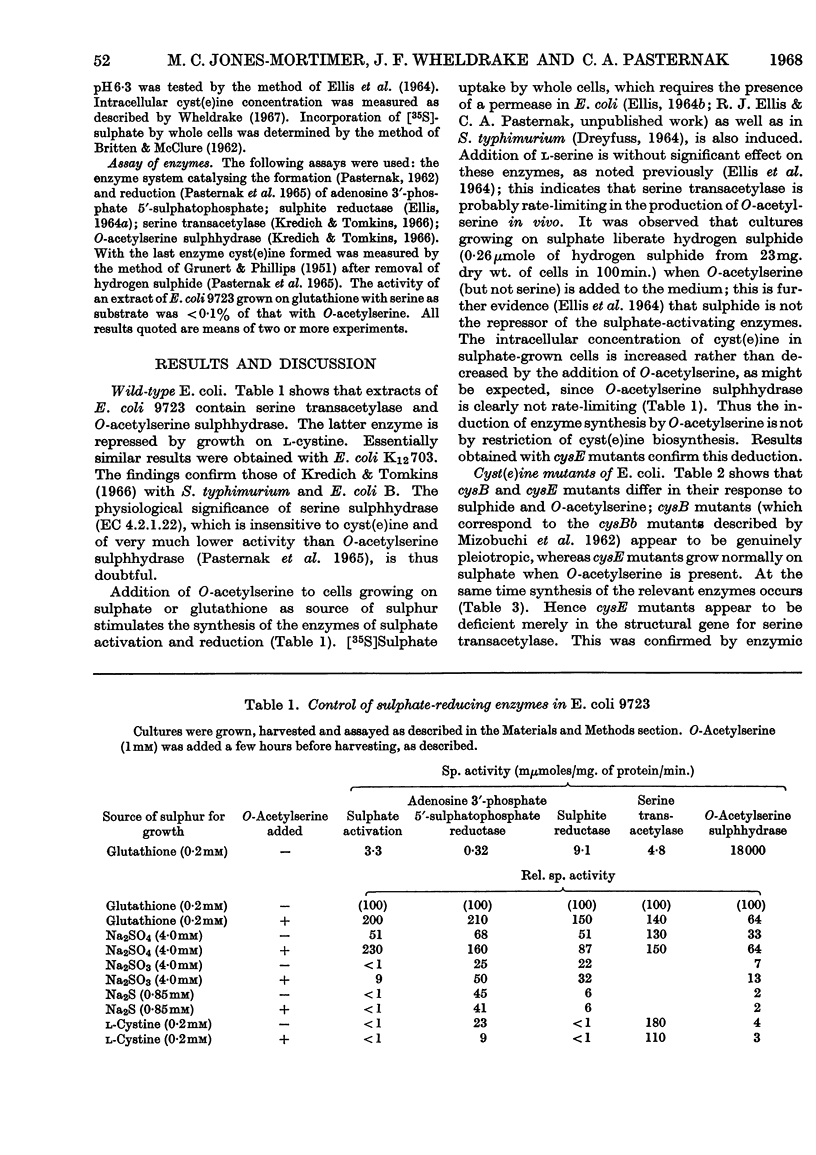
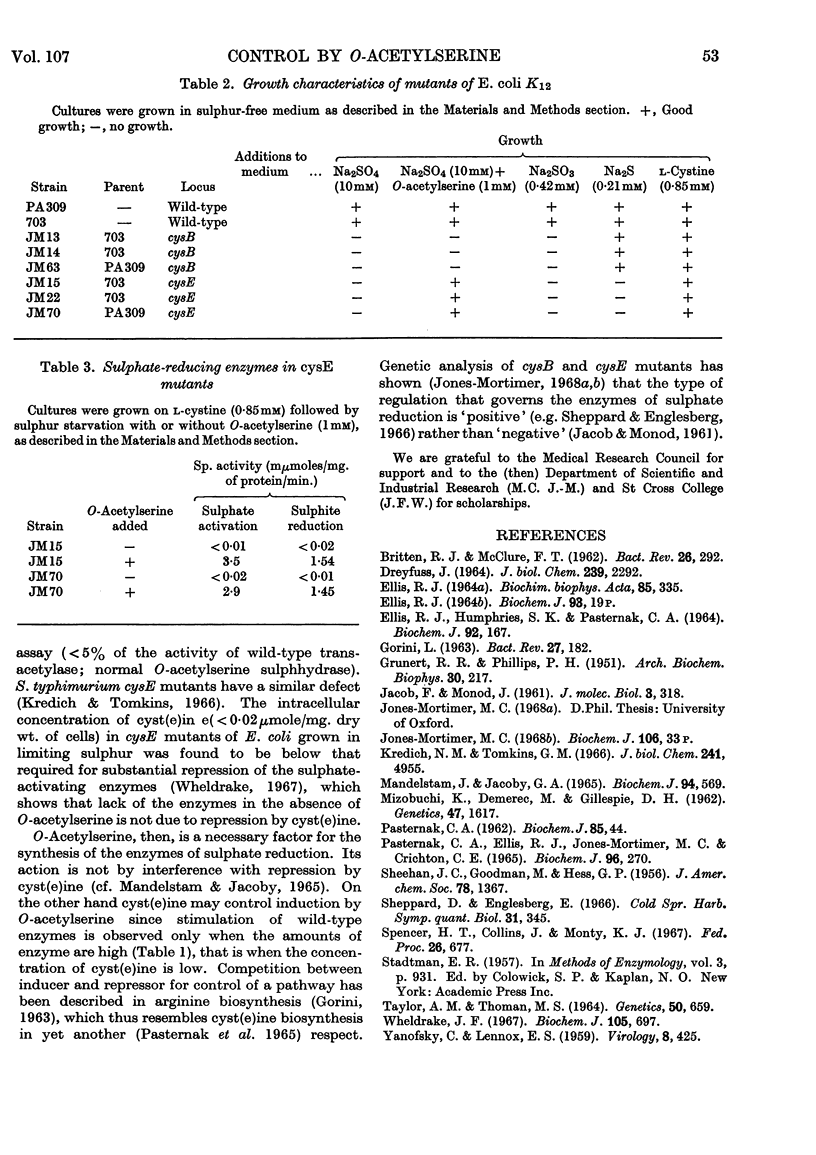
1. Extracts of Escherichia coli A.T.C.C. 9723 and K12703 contain serine transacetylase and O-acetylserine sulphhydrase. Synthesis of the latter enzyme is repressed by growth on l-cyst(e)ine and other sulphur compounds. 2. O-Acetyl-l-serine added to cells growing on glutathione or sulphate as source of sulphur induces the enzymes that catalyse (a) the activation of sulphate to adenosine 3′-phosphate 5′-sulphatophosphate (EC 2.7.7.4 and 2.7.1.25), (b) the reduction of adenosine 3′-phosphate 5′-sulphatophosphate to sulphite and (c) the reduction of sulphite to sulphide (EC 1.8.1.2). Hydrogen sulphide is liberated from cultures growing on sulphate as source of sulphur and in the presence of O-acetylserine. 3. The cysE mutants of E. coli K12 lack serine transacetylase. Addition of O-acetylserine permits growth on sulphate as source of sulphur; at the same time the enzymes of sulphate reduction, previously absent, are synthesized. Such mutants have no detectable intracellular cyst(e)ine when starved of sulphur. 4. These results suggest that O-acetylserine is necessary for synthesizing the enzymes of sulphate reduction in E. coli. Its action does not appear to be by interference with the repressive control exerted over these enzymes by cyst(e)ine.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BRITTEN R. J., McCLURE F. T. The amino acid pool in Escherichia coli. Bacteriol Rev. 1962 Sep;26:292–335. doi: 10.1128/br.26.3.292-335.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DREYFUSS J. CHARACTERIZATION OF A SULFATE- AND THIOSULFATE-TRANSPORTING SYSTEM IN SALMONELLA TYPHIMURIUM. J Biol Chem. 1964 Jul;239:2292–2297. [PubMed] [Google Scholar]
- ELLIS R. J. A RAPID ASSAY FOR SULPHITE REDUCTASE. Biochim Biophys Acta. 1964 May 4;85:335–338. doi: 10.1016/0926-6569(64)90255-x. [DOI] [PubMed] [Google Scholar]
- Ellis R. J., Humphries S. K., Pasternak C. A. Repressors of sulphate activation in Escherichia coli. Biochem J. 1964 Jul;92(1):167–172. doi: 10.1042/bj0920167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GORINI L. Symposium on multiple forms of enzymes and control mechanisms. III. Control by repression of a biochemical pathway. Bacteriol Rev. 1963 Jun;27:182–190. doi: 10.1128/br.27.2.182-190.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GRUNERT R. R., PHILLIPS P. H. A modification of the nitroprusside method of analysis for glutathione. Arch Biochem. 1951 Feb;30(2):217–225. [PubMed] [Google Scholar]
- JACOB F., MONOD J. Genetic regulatory mechanisms in the synthesis of proteins. J Mol Biol. 1961 Jun;3:318–356. doi: 10.1016/s0022-2836(61)80072-7. [DOI] [PubMed] [Google Scholar]
- Kredich N. M., Tomkins G. M. The enzymic synthesis of L-cysteine in Escherichia coli and Salmonella typhimurium. J Biol Chem. 1966 Nov 10;241(21):4955–4965. [PubMed] [Google Scholar]
- MANDELSTAM J., JACOBY G. A. INDUCTION AND MULTI-SENSITIVE END-PRODUCT REPRESSION IN THE ENZYMIC PATHWAY DEGRADING MANDELATE IN PSEUDOMONAS FLUORESCENS. Biochem J. 1965 Mar;94:569–577. doi: 10.1042/bj0940569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizobuchi K, Demerec M, Gillespie D H. Cysteine Mutants of Salmonella Typhimurium. Genetics. 1962 Nov;47(11):1617–1627. doi: 10.1093/genetics/47.11.1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PASTERNAK C. A., ELLIS R. J., JONES-MORTIMER M. C., CRICHTON C. E. THE CONTROL OF SULPHATE REDUCTION IN BACTERIA. Biochem J. 1965 Jul;96:270–275. doi: 10.1042/bj0960270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PASTERNAK C. A. Sulphate activation and its control in Escherichia coli and Bacillus subtilis. Biochem J. 1962 Oct;85:44–49. doi: 10.1042/bj0850044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheppard D., Englesberg E. Positive control in the L-arabinose gene-enzyme complex of Escherichia coli B/r exhibited with stable merodiploids. Cold Spring Harb Symp Quant Biol. 1966;31:345–347. doi: 10.1101/sqb.1966.031.01.044. [DOI] [PubMed] [Google Scholar]
- TAYLOR A. L., THOMAN M. S. THE GENETIC MAP OF ESCHERICHIA COLI K-12. Genetics. 1964 Oct;50:659–677. doi: 10.1093/genetics/50.4.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheldrake J. F. Intracellular concentration of cysteine in Escherichia coli and its relation to repression of the sulphate-activating enzymes. Biochem J. 1967 Nov;105(2):697–699. doi: 10.1042/bj1050697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YANOFSKY C., LENNOX E. S. Transduction and recombination study of linkage relationships among the genes controlling tryptophan synthesis in Escherichia coli. Virology. 1959 Aug;8:425–447. doi: 10.1016/0042-6822(59)90046-7. [DOI] [PubMed] [Google Scholar]


