Abstract
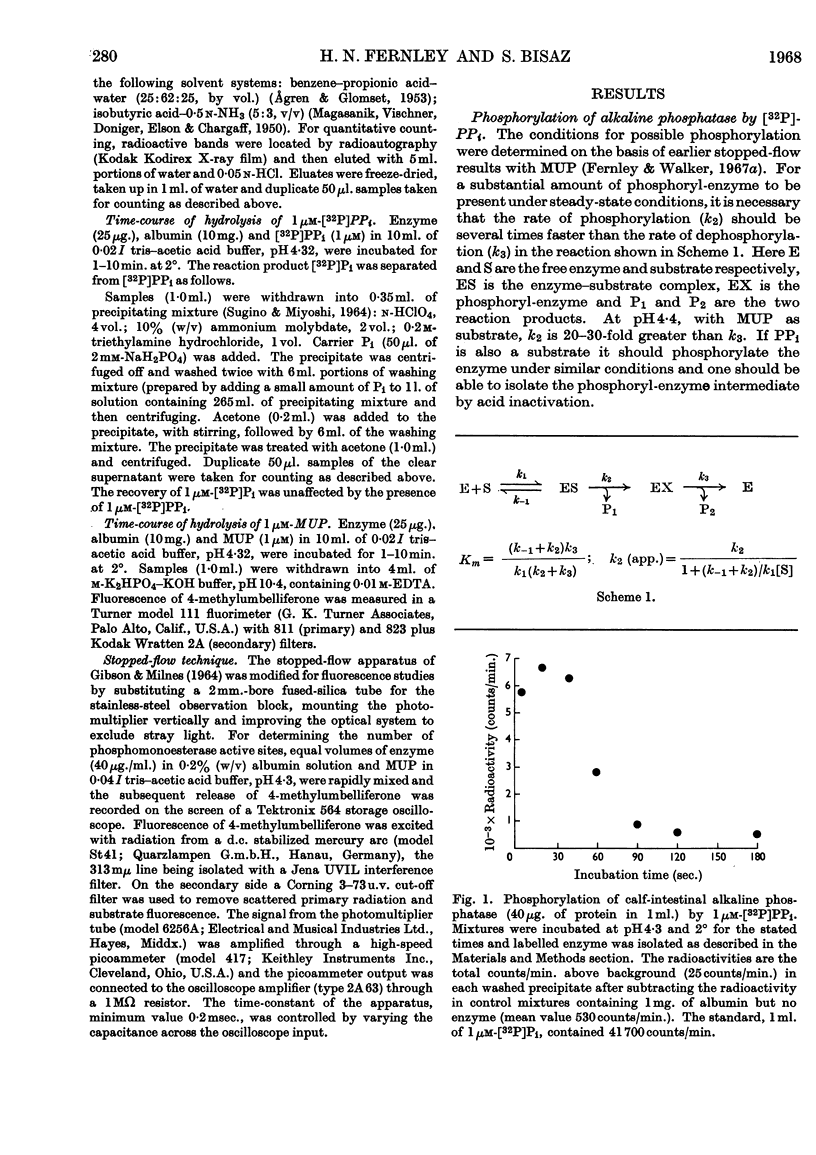
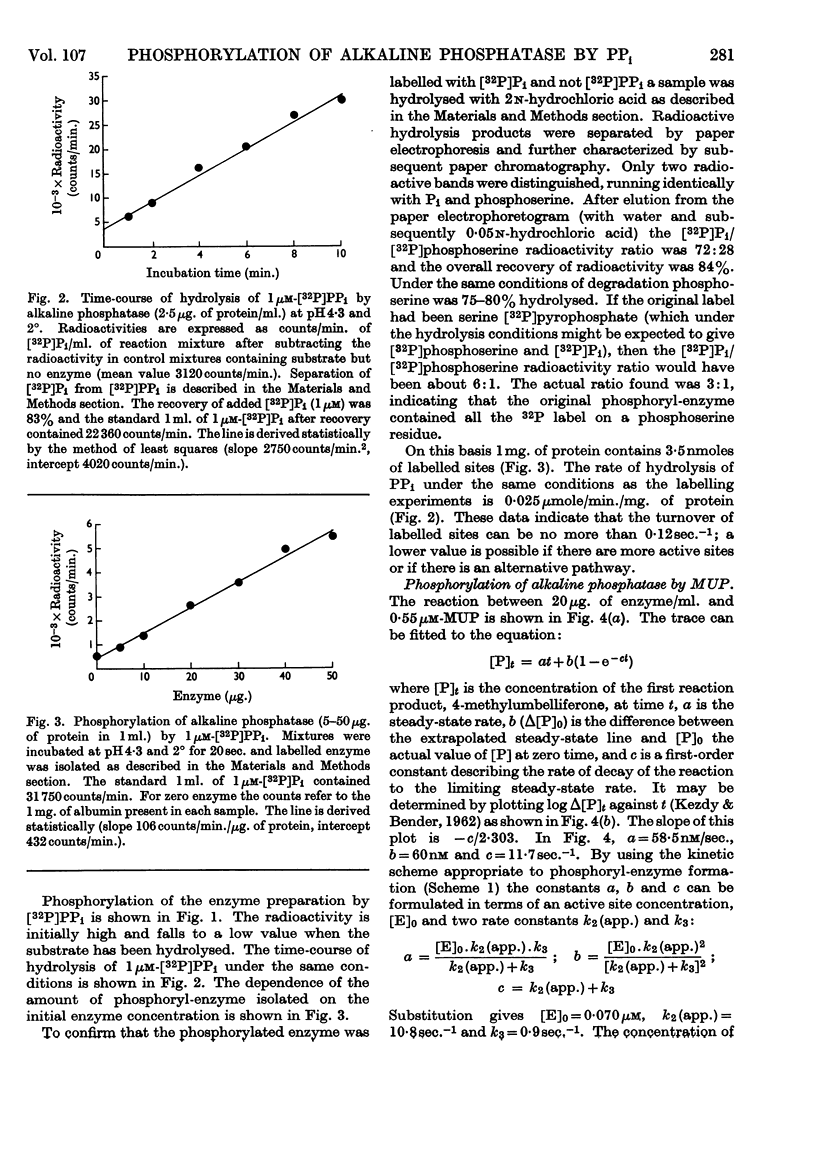
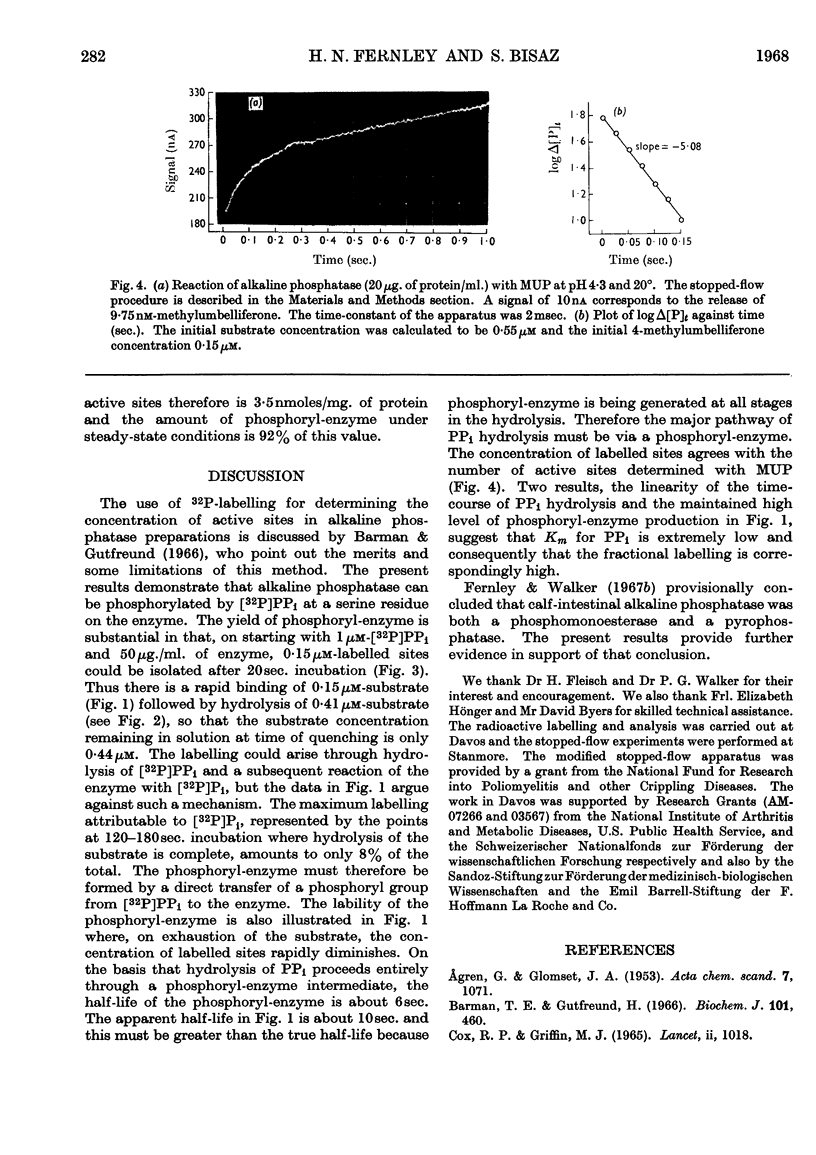
1. A purified preparation of alkaline phosphatase from calf-intestinal mucosa was phosphorylated by 32P-labelled PPi at a serine residue on the enzyme. Under the conditions employed, up to 0·15μm-labelled sites were obtained from 1μm-[32P]PPi. 2. The phosphorylated enzyme was labile, the rate of dephosphorylation being similar to the overall rate of substrate hydrolysis. 3. A stopped-flow technique was used to determine the number of phosphomonoesterase active sites, which agreed with the number of 32P-labelled sites. 4. It is concluded that calf-intestinal alkaline phosphatase is both a phosphomonoesterase and a pyrophosphatase.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barman T. E., Gutfreund H. The catalytic-centre activity and kinetic properties of bovine milk alkaline phosphatase. Biochem J. 1966 Nov;101(2):460–466. doi: 10.1042/bj1010460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernley H. N., Walker P. G. Kinetic behaviour of calf-intestinal alkaline phosphatase with 4-methylumbelliferyl phosphate. Biochem J. 1965 Oct;97(1):95–103. doi: 10.1042/bj0970095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernley H. N., Walker P. G. Studies on alkaline phosphatase. Inhibition by phosphate derivatives and the substrate specificity. Biochem J. 1967 Sep;104(3):1011–1018. doi: 10.1042/bj1041011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson Q. H., Milnes L. Apparatus for rapid and sensitive spectrophotometry. Biochem J. 1964 Apr;91(1):161–171. doi: 10.1042/bj0910161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KEZDY F. J., BENDER M. L. The kinetics of the alpha-chymotrypsin-catalyzed hydrolysis of p-nitrophenyl acetate. Biochemistry. 1962 Nov;1:1097–1106. doi: 10.1021/bi00912a021. [DOI] [PubMed] [Google Scholar]
- MAGASANIK B., VISCHER E., DONIGER R., ELSON D., CHARGAFF E. The separation and estimation of ribonucleotides in minute quantities. J Biol Chem. 1950 Sep;186(1):37–50. [PubMed] [Google Scholar]
- MORTON R. K. The substrate specificity and inhibition of alkaline phosphatases of cow's milk and calf intestinal mucosa. Biochem J. 1955 Oct;61(2):232–240. doi: 10.1042/bj0610232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss D. W., Eaton R. H., Smith J. K., Whitby L. G. Association of inorganic-pyrophosphatase activity with human alkaline-phosphatase preparations. Biochem J. 1967 Jan;102(1):53–57. doi: 10.1042/bj1020053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rochman H., Lathe G. H., Levell M. J. The effect of temperature and anoxia of kidney on the subsequent oxidative phosphorylation of mitochondria. Biochem J. 1967 Jan;102(1):48–52. doi: 10.1042/bj1020048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SUGINO Y., MIYOSHI Y. THE SPECIFIC PRECIPITATION OF ORTHOPHOSPHATE AND SOME BIOCHEMICAL APPLICATIONS. J Biol Chem. 1964 Jul;239:2360–2364. [PubMed] [Google Scholar]


