Abstract
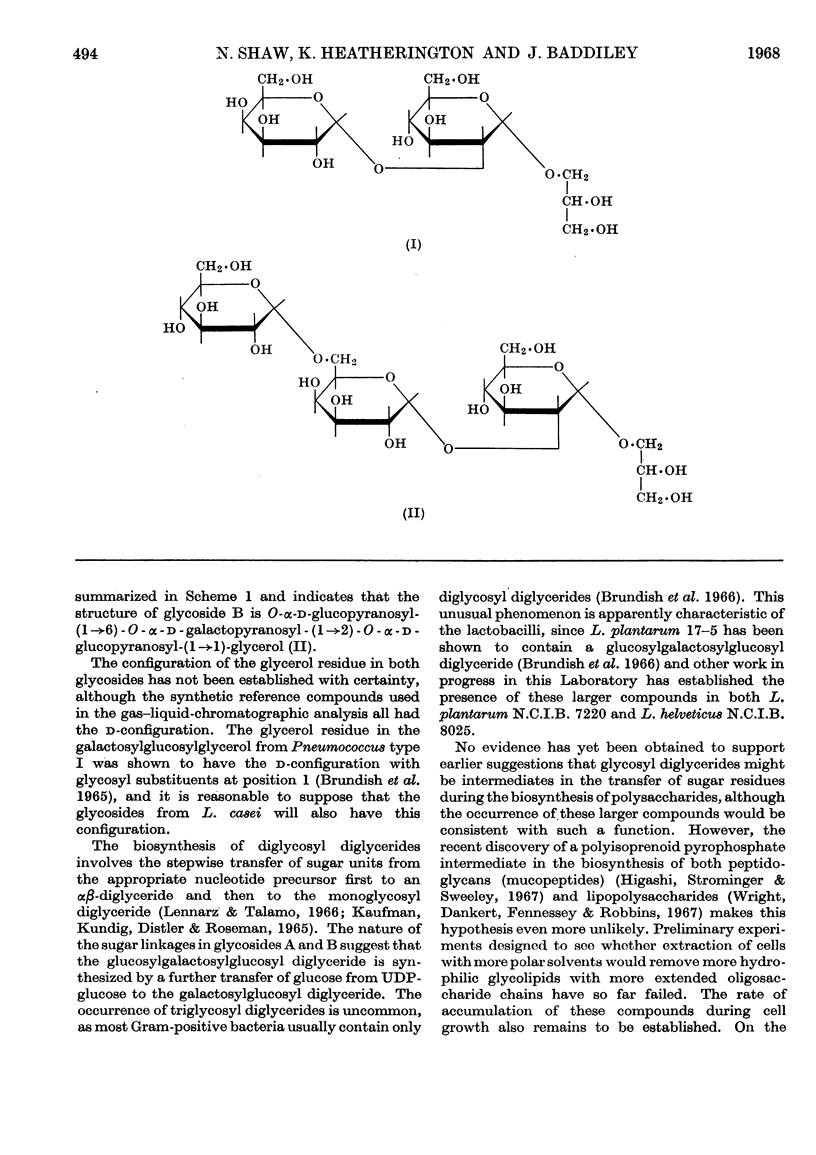
1. The lipids were extracted from Lactobacillus casei A.T.C.C. 7469 with chloroform–methanol mixtures. The glycolipids were obtained by chromatography on silicic acid and DEAE-cellulose (acetate form). 2. Hydrolysis of the glycolipids with alkali gave two glycerol glycosides and a mixture of fatty acids. 3. The glycosides were separated and their structures elucidated. The major component was O-α-d-galactopyranosyl-(1→2)-O-α-d-glucopyranosyl-(1→1)-glycerol and the minor component O-α-d-glucopyranosyl-(1→6)-O-α-d-galactopyranosyl-(1→2)-O-α-d-glucopyranosyl-(1→1)-glycerol. 4. Analysis of the fatty acids by gas–liquid chromatography showed that they were predominantly palmitic acid, octadecenoic acid and lactobacillic acid.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brundish D. E., Shaw N., Baddiley J. Bacterial glycolipids. Glycosyl diglycerides in gram-positive bacteria. Biochem J. 1966 Jun;99(3):546–549. doi: 10.1042/bj0990546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brundish D. E., Shaw N., Baddiley J. The glycolipids from the non-capsulated strain of Pneumococcus I-192R, A.T.C.C. 12213. Biochem J. 1965 Oct;97(1):158–165. doi: 10.1042/bj0970158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brundish D. E., Shaw N., Baddiley J. The structure and possible function of the glycolipid from Staphylococcus lactis I3. Biochem J. 1967 Nov;105(2):885–889. doi: 10.1042/bj1050885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DITTMER J. C., LESTER R. L. A SIMPLE, SPECIFIC SPRAY FOR THE DETECTION OF PHOSPHOLIPIDS ON THIN-LAYER CHROMATOGRAMS. J Lipid Res. 1964 Jan;5:126–127. [PubMed] [Google Scholar]
- HANAHAN D. J., OLLEY J. N. Chemical nature of monophosphoinositides. J Biol Chem. 1958 Apr;231(2):813–828. [PubMed] [Google Scholar]
- Higashi Y., Strominger J. L., Sweeley C. C. Structure of a lipid intermediate in cell wall peptidoglycan synthesis: a derivative of a C55 isoprenoid alcohol. Proc Natl Acad Sci U S A. 1967 Jun;57(6):1878–1884. doi: 10.1073/pnas.57.6.1878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KAUFMAN B., KUNDIG D., DISTLER J., ROSEMAN S. ENZYMATIC SYNTHESIS AND STRUCTURE OF TWO GLYCOLIPIDS FROM TYPE XIV PNEUMOCOCCUS. Biochem Biophys Res Commun. 1965 Feb 3;18:312–318. doi: 10.1016/0006-291x(65)90705-9. [DOI] [PubMed] [Google Scholar]
- Lennarz W. J., Talamo B. The chemical characterization and enzymatic synthesis of mannolipids in Micrococcus lysodeikticus. J Biol Chem. 1966 Jun 10;241(11):2707–2719. [PubMed] [Google Scholar]
- Roberts W. K., Buchanan J. G., Baddiley J. The specific substance from Pneumococcus type 34 (41). The structure of a phosphorus-free repeating unit. Biochem J. 1963 Jul;88(1):1–7. doi: 10.1042/bj0880001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- THORNE K. J., KODICEK E. The metabolism of acetate and mevalonic acid by lactobacilli. IV. Analysis of the fatty acids by gas-liquid chromatography. Biochim Biophys Acta. 1962 May 21;59:306–312. doi: 10.1016/0006-3002(62)90178-6. [DOI] [PubMed] [Google Scholar]
- TREVELYAN W. E., PROCTER D. P., HARRISON J. S. Detection of sugars on paper chromatograms. Nature. 1950 Sep 9;166(4219):444–445. doi: 10.1038/166444b0. [DOI] [PubMed] [Google Scholar]
- VORBECK M. L., MARINETTI G. V. SEPARATION OF GLYCOSYL DIGLYCERIDES FROM PHOSPHATIDES USING SILICIC ACID COLUMN CHROMATOGRAPHY. J Lipid Res. 1965 Jan;6:3–6. [PubMed] [Google Scholar]
- WAGNER H., HOERHAMMER L., WOLFF P. [Thin layer chromatography of phosphatides and glycolipids]. Biochem Z. 1961;334:175–184. [PubMed] [Google Scholar]
- WELLS M. A., DITTMER J. C. THE USE OF SEPHADEX FOR THE REMOVAL OF NONLIPID CONTAMINANTS FROM LIPID EXTRACTS. Biochemistry. 1963 Nov-Dec;2:1259–1263. doi: 10.1021/bi00906a015. [DOI] [PubMed] [Google Scholar]
- Wright A., Dankert M., Fennessey P., Robbins P. W. Characterization of a polyisoprenoid compound functional in O-antigen biosynthesis. Proc Natl Acad Sci U S A. 1967 Jun;57(6):1798–1803. doi: 10.1073/pnas.57.6.1798. [DOI] [PMC free article] [PubMed] [Google Scholar]


