Abstract
`Fingerprints' of bovine colostrum and serum immunoglobulin G1 heavy chains were extremely similar, but different from serum immunoglobin G2 heavy chains. Serum immunoglobulin G1 and immunoglobulin G2 heavy chains were treated with cyanogen bromide. The fractions from the C-terminal end of the heavy chains were isolated and the amino acid sequence of this fraction from immunoglobulin G2 was:His-Glx-Ala-Leu-His-Asx-His-Tyr-Met-Gln-Lys-Ser-Thr-Ser-Lys-Ser-Ala-GlyThe amino acid composition of this fraction from immunoglobulin G1 was the same except for the methionine, which in immunoglobulin G1 was replaced by threonine.
Full text
PDF
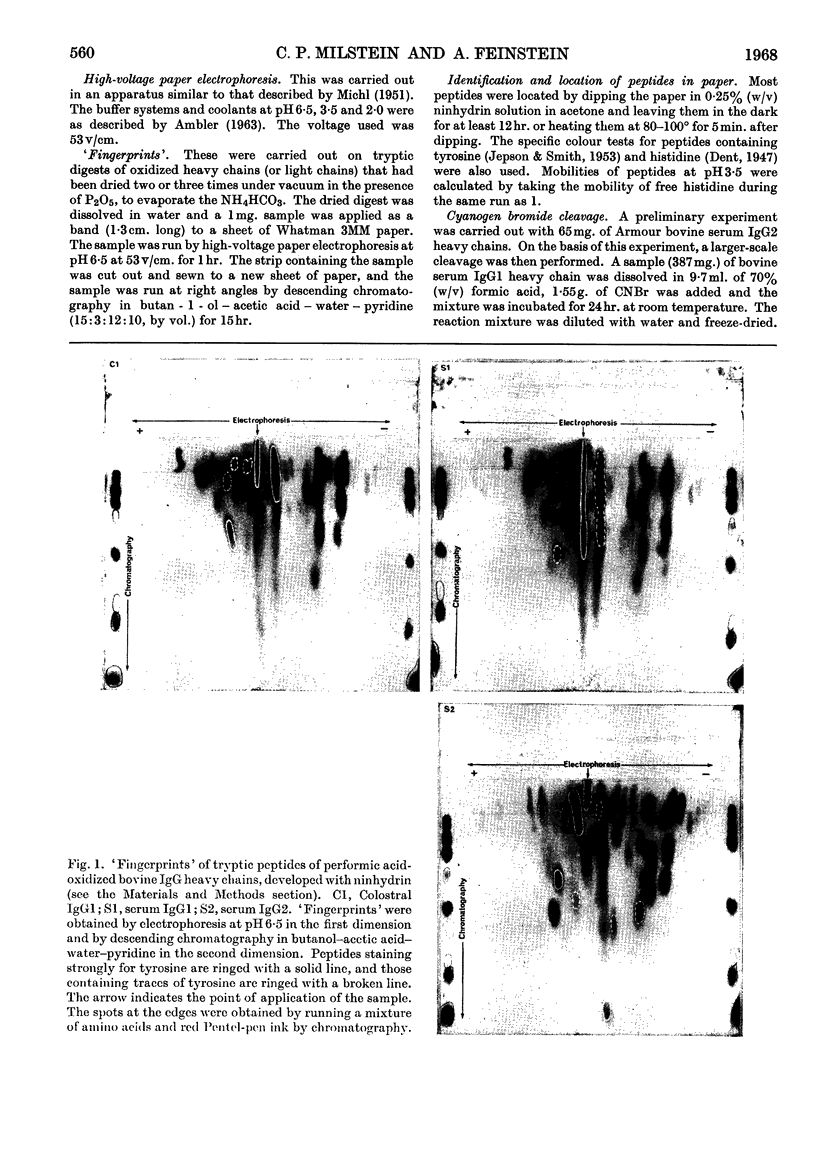

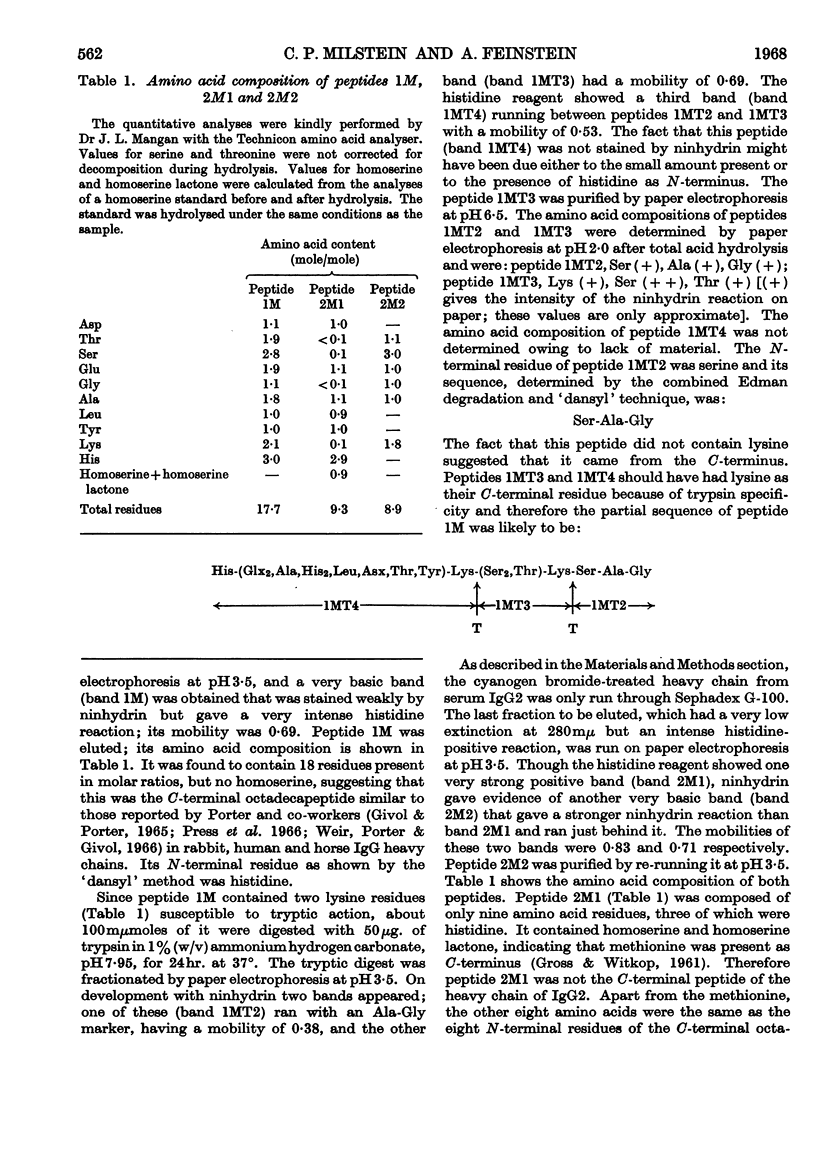


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AMBLER R. P. THE AMINO ACID SEQUENCE OF PSEUDOMONAS CYTOCHROME C-551. Biochem J. 1963 Nov;89:349–378. doi: 10.1042/bj0890349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cebra J. J., Small P. A., Jr Polypeptide chain structure of rabbit immunoglobulins. 3. Secretory gamma-A-immunoglobulin from colostrum. Biochemistry. 1967 Feb;6(2):503–512. doi: 10.1021/bi00854a019. [DOI] [PubMed] [Google Scholar]
- Crowshaw K., Jessup S. J., Ramwell P. W. Thin-layer chromatography of 1-dimethylaminonaphthalene-5-sulphonyl derivatives of amino acids present in superfusates of cat cerebral cortex. Biochem J. 1967 Apr;103(1):79–85. doi: 10.1042/bj1030079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dent C. E. The amino-aciduria in Fanconi syndrome. A study making extensive use of techniques based on paper partition chromatography. Biochem J. 1947;41(2):240–253. doi: 10.1042/bj0410240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FLEISCHMAN J. B., PAIN R. H., PORTER R. R. Reduction of gamma-globulins. Arch Biochem Biophys. 1962 Sep;Suppl 1:174–180. [PubMed] [Google Scholar]
- Hong R., Pollara B., Good R. A. A model for colostral IgA. Proc Natl Acad Sci U S A. 1966 Aug;56(2):602–607. doi: 10.1073/pnas.56.2.602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JEPSON J. B., SMITH I. Multiple dipping procedures in paper chromatography: a specific test for hydroxy-proline. Nature. 1953 Dec 12;172(4389):1100–1101. doi: 10.1038/1721100b0. [DOI] [PubMed] [Google Scholar]
- Offord R. E. Electrophoretic mobilities of peptides on paper and their use in the determination of amide groups. Nature. 1966 Aug 6;211(5049):591–593. doi: 10.1038/211591a0. [DOI] [PubMed] [Google Scholar]
- PIERCE A. E., FEINSTEIN A. BIOPHYSICAL AND IMMUNOLOGICAL STUDIES ON BOVINE IMMUNE GLOBULINS WITH EVIDENCE FOR SELECTIVE TRANSPORT WITHIN THE MAMMARY GLAND FROM MATERNAL PLASMA TO COLOSTRUM. Immunology. 1965 Jan;8:106–123. [PMC free article] [PubMed] [Google Scholar]
- Press E. M., Piggot P. J., Porter R. R. The N- and c-terminal amino acid sequences of the heavy chain from a pathological human immunoglobulin IgG. Biochem J. 1966 May;99(2):356–366. doi: 10.1042/bj0990356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weir R. C., Porter R. R., Givol D. Comparison of the C-terminal amino-acid sequence of two horse immunoglobulins IgG and IgG(T). Nature. 1966 Oct 8;212(5058):205–206. doi: 10.1038/212205a0. [DOI] [PubMed] [Google Scholar]
- Woods K. R., Wang K. T. Separation of dansyl-amino acids by polyamide layer chromatography. Biochim Biophys Acta. 1967 Feb 21;133(2):369–370. doi: 10.1016/0005-2795(67)90078-5. [DOI] [PubMed] [Google Scholar]