Abstract
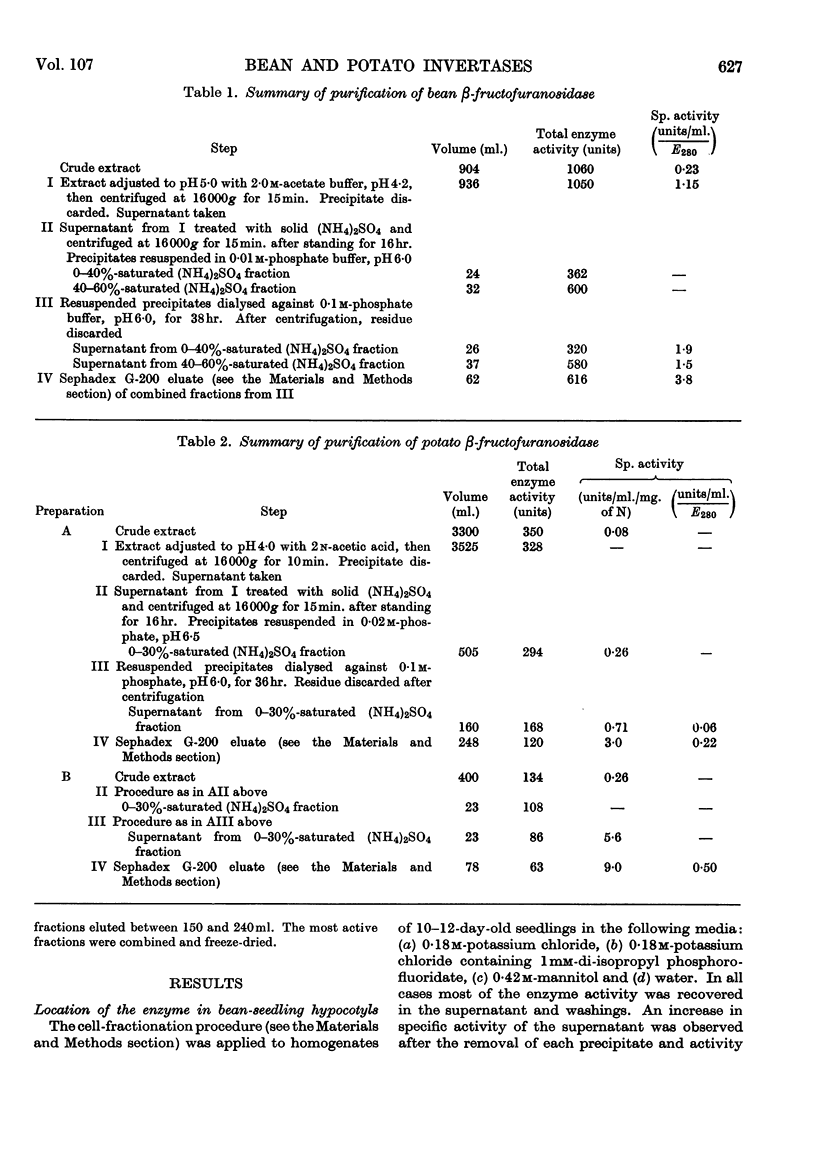
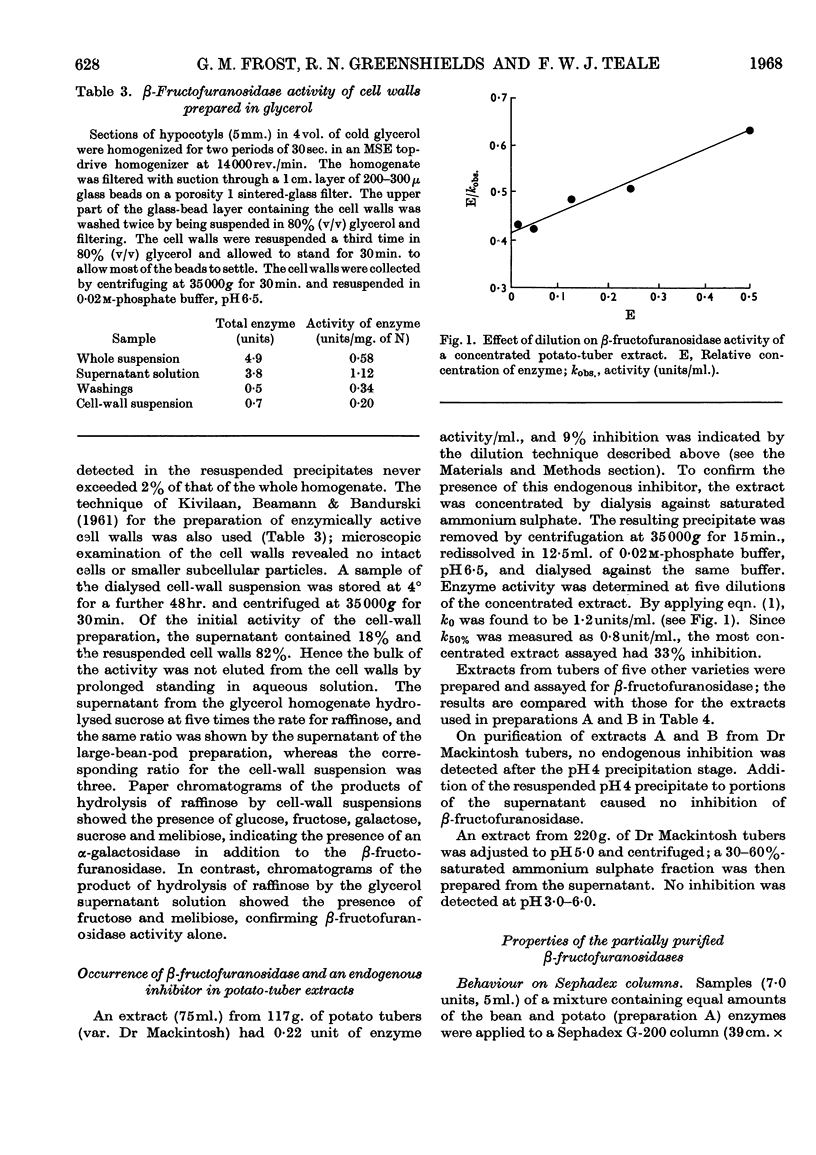
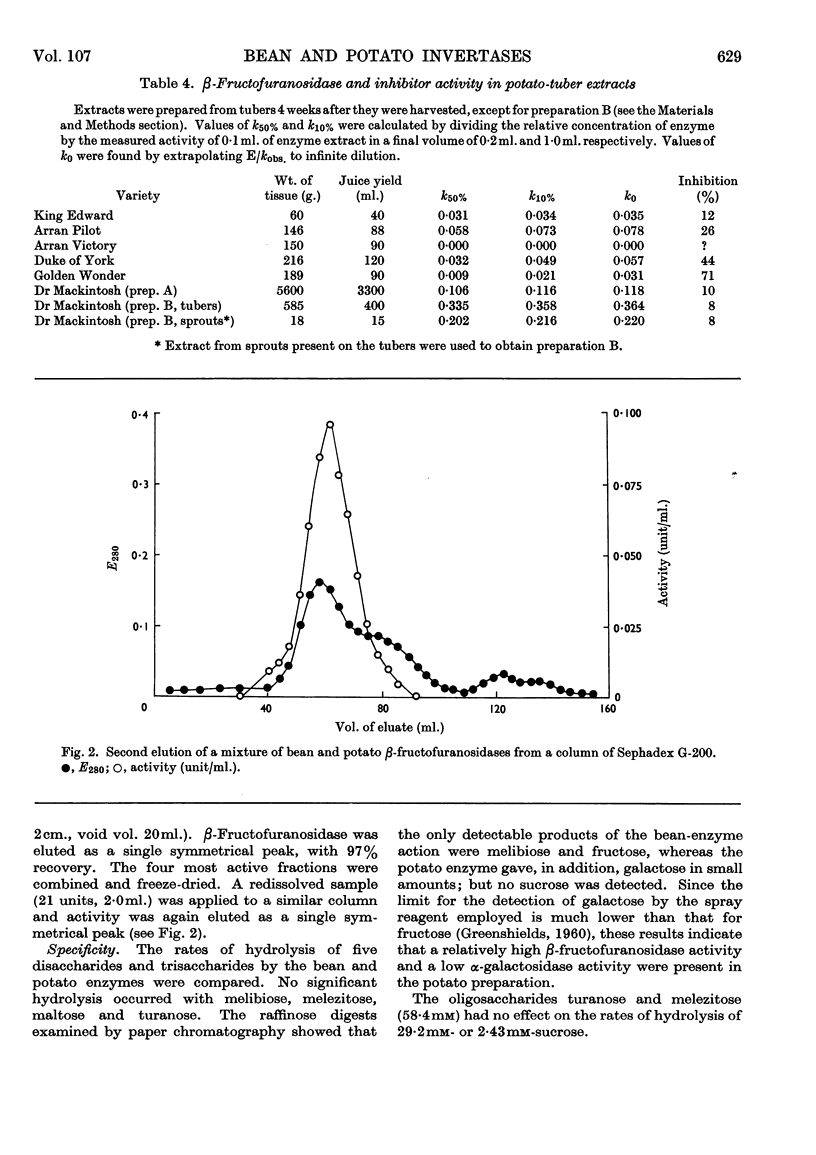
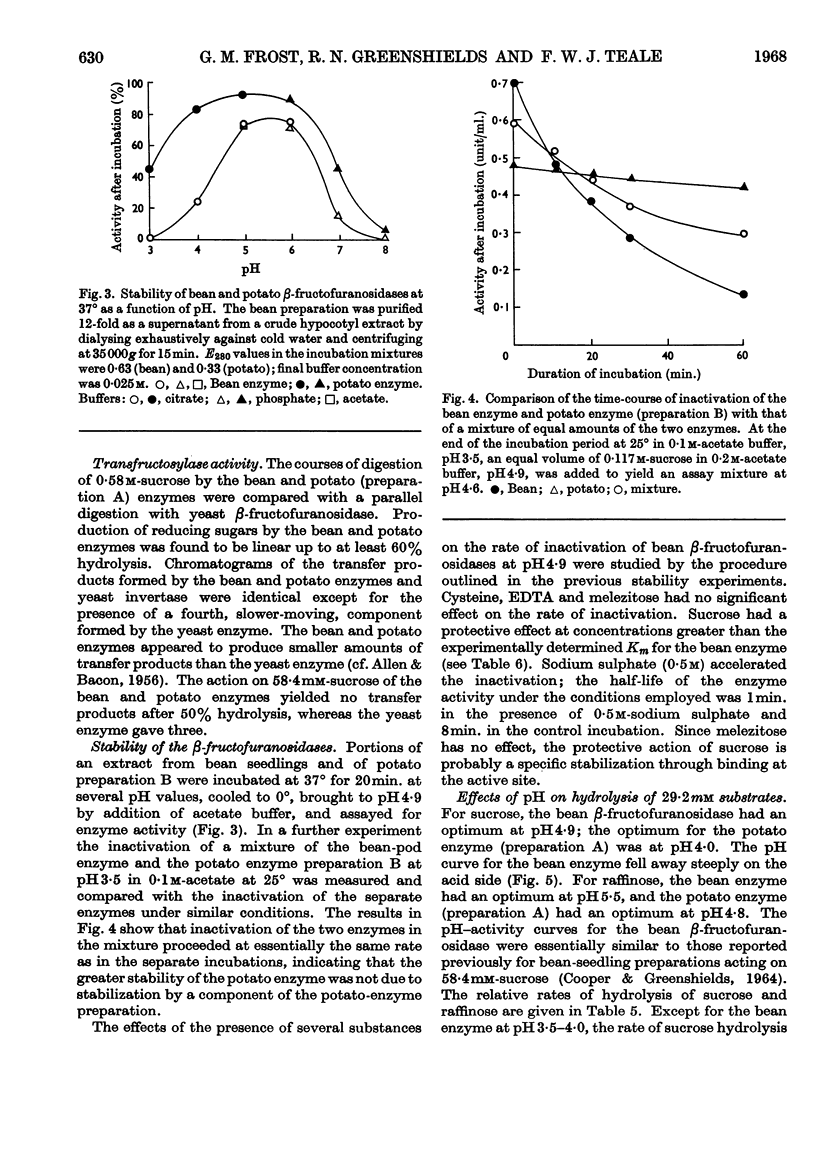
1. Soluble β-fructofuranosidases were purified 16-fold from French-bean-pod extracts and 35-fold from potato-tuber extracts. 2. The two enzymes had similar overall properties but differed from each other quantitatively in lability and reaction kinetics. 3. A non-diffusible inhibitor of β-fructofuranosidase was present in the potato-tuber extracts but was absent from the bean extracts. This non-competitive inhibitor was acid-labile. 4. The possible roles of imidazole, carboxyl and thiol groups in β-fructofuranosidase action are discussed, and the properties of the bean and potato enzymes are compared with published data for yeast, mould and grape-berry enzymes.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALLEN P. J., BACON J. S. Oligosaccharides formed from sucrose by fructose-transferring enzymes of higher plants. Biochem J. 1956 Jun;63(2):200–206. doi: 10.1042/bj0630200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold W. N. A column method for enzymic characterization of coarse cellular fractions: application to insoluble beta-fructofuranosidase from grape. Arch Biochem Biophys. 1966 Feb;113(2):451–456. doi: 10.1016/0003-9861(66)90213-x. [DOI] [PubMed] [Google Scholar]
- Arnold W. N. Beta-fructofuranosidase from grape berries. Biochim Biophys Acta. 1965 Oct 25;110(1):134–147. doi: 10.1016/s0926-6593(65)80102-3. [DOI] [PubMed] [Google Scholar]
- BACON J. S. D., EDELMAN J. The carbohydrates of the Jerusalem artichoke and other Compositae. Biochem J. 1951 Jan;48(1):114–126. doi: 10.1042/bj0480114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BEALING F. J., BACON J. S. D. The action of mould enzymes on sucrose. Biochem J. 1953 Jan;53(2):277–285. doi: 10.1042/bj0530277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COOPER R. A., GREENSHIELDS R. N. Sucrases in Phaseolus vulgaris. Nature. 1961 Aug 5;191:601–602. doi: 10.1038/191601b0. [DOI] [PubMed] [Google Scholar]
- Cooper R. A., Greenshields R. N. The partial purification and some properties of two sucrases of Phaseolus vulgaris. Biochem J. 1964 Aug;92(2):357–364. doi: 10.1042/bj0920357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAHLQVIST A. Determination of maltase and isomaltase activities with a glucose-oxidase reagent. Biochem J. 1961 Sep;80:547–551. doi: 10.1042/bj0800547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DIXON M. The effect of pH on the affinities of enzymes for substrates and inhibitors. Biochem J. 1953 Aug;55(1):161–170. doi: 10.1042/bj0550161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EDELMAN J. Transfer reactions catalysed by some sucrase preparations. Biochem J. 1954 May;57(1):22–33. doi: 10.1042/bj0570022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GILLESPIE R. E., LARNER J. Gastrointestinal digestion of starch. II. Properties of the intestinal carbohydrases. J Biol Chem. 1956 Dec;223(2):709–726. [PubMed] [Google Scholar]
- HENDERSON R. W., MORTON R. K., RAWLINSON W. A. Oligosaccharide synthesis in the banana and its relationship to the transferase activity of invertase. Biochem J. 1959 Jun;72(2):340–344. doi: 10.1042/bj0720340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatch M. D., Sacher J. A., Glasziou K. T. Sugar Accumulation Cycle in Sugar Cane. I. Studies on Enzymes of the Cycle. Plant Physiol. 1963 May;38(3):338–343. doi: 10.1104/pp.38.3.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KOSHLAND D. E., Jr, RAY W. J., Jr, ERWIN M. J. Protein structure and enzyme action. Fed Proc. 1958 Dec;17(4):1145–1150. [PubMed] [Google Scholar]
- Kivilaan A., Beaman T. C., Bandurski R. S. Enzymatic activities associated with cell wall preparations from corn coleoptiles. Plant Physiol. 1961 Sep;36(5):605–610. doi: 10.1104/pp.36.5.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LARNER J., GILLESPIE R. E. Participation of histidine in catalytic activity of intestinal carbohydrases. Arch Biochem Biophys. 1955 Sep;58(1):252–253. doi: 10.1016/0003-9861(55)90117-x. [DOI] [PubMed] [Google Scholar]
- METZENBERG R. L. The purification and properties of invertase of Neurospora. Arch Biochem Biophys. 1963 Mar;100:503–511. doi: 10.1016/0003-9861(63)90118-8. [DOI] [PubMed] [Google Scholar]
- Neumann N. P., Lampen J. O. Purification and properties of yeast invertase. Biochemistry. 1967 Feb;6(2):468–475. doi: 10.1021/bi00854a015. [DOI] [PubMed] [Google Scholar]
- RABEK V., MANSFIELD V. Gel filtration of protease inhibitors from potatoes. Experientia. 1963 Mar 15;19:151–152. doi: 10.1007/BF02171605. [DOI] [PubMed] [Google Scholar]
- ROREM E. S., SCHWIMMER S. Double pH optima of potato invertase. Experientia. 1963 Mar 15;19:150–151. doi: 10.1007/BF02171604. [DOI] [PubMed] [Google Scholar]
- RUCHTI J., MCLAREN A. D. ENZYME REACTIONS IN STRUCTURALLY RESTRICTED SYSTEMS V. FURTHER OBSERVATIONS ON THE KINETICS OF YEAST BETA-FRUCTOFURANOSIDASE (INVERTASE) ACTIVITY IN VISCOUS MEDIA. Enzymologia. 1964 Aug 15;27:185–198. [PubMed] [Google Scholar]
- RYAN C. A., BALLS A. K. An inhibitor of chymotrypsin from Solanum tuberosm and its behavior toward trypsin. Proc Natl Acad Sci U S A. 1962 Oct 15;48:1839–1844. doi: 10.1073/pnas.48.10.1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwimmer S., Makower R. U., Rorem E. S. Invertase & invertase inhibitor in potato. Plant Physiol. 1961 May;36(3):313–316. doi: 10.1104/pp.36.3.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- THOMA J. A., WAKIM J., STEWART L. COMPARISON OF THE ACTIVE SITES OF ALPHA AND BETA AMYLASES. Biochem Biophys Res Commun. 1963 Aug 14;12:350–355. doi: 10.1016/0006-291x(63)90103-7. [DOI] [PubMed] [Google Scholar]
- TREVITHICK J. R., METZENBERG R. L. KINETICS OF THE INHIBITION OF NEUROSPORA INVERTASE BY PRODUCTS AND ANILINE. Arch Biochem Biophys. 1964 Aug;107:260–270. doi: 10.1016/0003-9861(64)90328-5. [DOI] [PubMed] [Google Scholar]
- WHITE J. W., Jr, SUBERS M. H. A glucose oxidase reagent for maltase assay. Anal Biochem. 1961 Aug;2:380–384. doi: 10.1016/0003-2697(61)90011-2. [DOI] [PubMed] [Google Scholar]


