Abstract
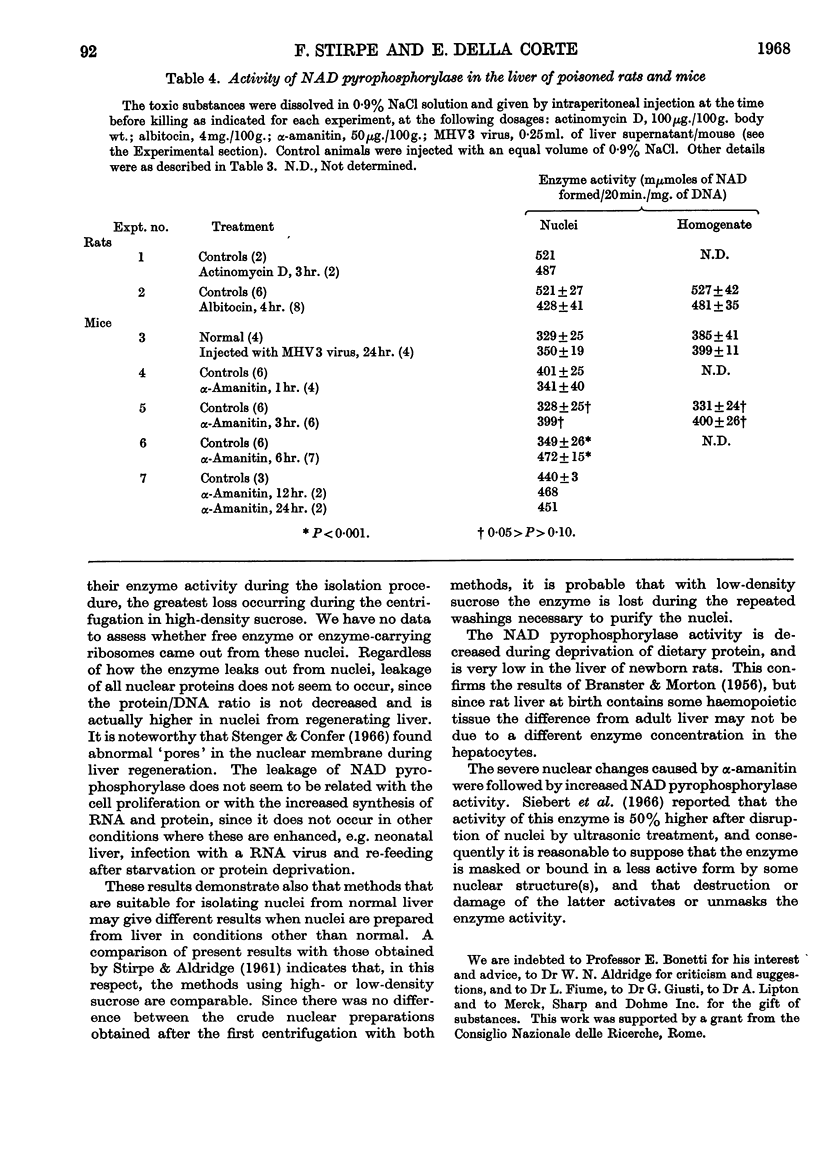
1. The activity of NAD pyrophosphorylase is lower in nuclei isolated from regenerating rat liver than in normal nuclei, and this is due to leakage of the enzyme from the nuclei during the isolation. 2. The NAD pyrophosphorylase activity is lower in liver nuclei from newborn rats, and from rats on a protein-free diet, but no leakage occurs in these cases. 3. Poisoning with α-amanitin brings about a transient enhancement of NAD pyrophosphorylase activity in mouse liver nuclei. 4. No changes of enzyme activity were observed after 72hr. starvation, administration of actinomycin D or infection with MHV3 virus.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BONETTI E., STIRPE F. Conditions affecting dietary liver necrosis and liver regeneration in rats. J Nutr. 1962 Jun;77:179–186. doi: 10.1093/jn/77.2.179. [DOI] [PubMed] [Google Scholar]
- BRANSTER M. V., MORTON R. K. Comparative rates of synthesis of diphosphopyridine nucleotide by normal and tumour tissue from mouse mammary gland; studies with isolated nuclei. Biochem J. 1956 Aug;63(4):640–646. doi: 10.1042/bj0630640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BURTON K. A study of the conditions and mechanism of the diphenylamine reaction for the colorimetric estimation of deoxyribonucleic acid. Biochem J. 1956 Feb;62(2):315–323. doi: 10.1042/bj0620315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHRISTIE G. S., BAILIE M. J., LE PAGE R. N. Acute toxic liver injury. nicotinamide-adenine dinucleotide-pyrophosphorylase activity of nuclei isolated from rat liver in heliotrine and in dimethylnitrosamine poisoning. Biochem J. 1962 Aug;84:364–368. doi: 10.1042/bj0840364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiume L., Laschi R. Lesioni ultrastrutturali prodotte nelle cellule parenchimali epatiche dalla falloidina e dalla alpha-amanitina. Sperimentale. 1965 Sep-Oct;115(5):288–297. [PubMed] [Google Scholar]
- GINZBURG-TIETZ Y., KAUFMANN E., TRAUB A. STUDIES ON NUCLEAR RIBOSOMES. II. TRANSFER OF RIBOSOMES FROM NUCLEUS TO CYTOPLASM IN THE EARLY STAGES OF VIRAL INFECTION. Exp Cell Res. 1964 Apr;34:384–395. doi: 10.1016/0014-4827(64)90373-8. [DOI] [PubMed] [Google Scholar]
- HOGEBOOM G. H., SCHNEIDER W. C. Cytochemical studies. VI. The synthesis of diphosphopyridine nucleotide by liver cell nuclei. J Biol Chem. 1952 May;197(2):611–620. [PubMed] [Google Scholar]
- JONES W. A., WARAVDEKAR V. S. DIPHOSPHYPYRIDINE NUCLEOTIDE-SYNTHESIZING ENZYME IN THE LIVER. Lab Invest. 1965 Apr;14:397–408. [PubMed] [Google Scholar]
- Kerr J. F., Pound A. W. Acute liver injury due to albitocin. Aust J Exp Biol Med Sci. 1966 Apr;44(2):197–204. doi: 10.1038/icb.1966.20. [DOI] [PubMed] [Google Scholar]
- LIPTON A. AN ACTIVE GLYCOSIDE FROM ALBIZIA SPECIES AND ITS ACTION ON ISOLATED UTERUS AND ILEUM. J Pharm Pharmacol. 1963 Dec;15:816–824. doi: 10.1111/j.2042-7158.1963.tb12886.x. [DOI] [PubMed] [Google Scholar]
- MYERS D. K. Effects of x-irradiation on enzyme synthesis during liver regeneration. Can J Biochem Physiol. 1962 May;40:619–630. [PubMed] [Google Scholar]
- Shimoyama M., Yamaguchi K., Gholson R. K. Enzymic lesions of nicotinamide adenine dinucleotide biosynthesis in hepatomas and in azo dye carcinogenesis. Cancer Res. 1967 Mar;27(3):578–583. [PubMed] [Google Scholar]
- Siebert G., Villalobos J., Jr, Ro T. S., Steele W. J., Lindenmayer G., Adams H., Busch H. Enzymatic studies on isolated nucleoli of rat liver. J Biol Chem. 1966 Jan 10;241(1):71–78. [PubMed] [Google Scholar]
- Stenger R. J., Confer D. B. Hepatocellular ultrastructure during liver regeneration after subtotal hepatectomy. Exp Mol Pathol. 1966 Oct;5(5):455–474. doi: 10.1016/0014-4800(66)90026-8. [DOI] [PubMed] [Google Scholar]
- Stirpe F., Aldridge W. N. Diphosphopyridine nucleotide pyrophosphorylase in the nuclei isolated from poisoned and regenerating rat liver. Biochem J. 1961 Sep;80(3):481–487. doi: 10.1042/bj0800481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stirpe F., Fiume L. Studies on the pathogenesis of liver necrosis by alpha-amanitin. Effect of alpha-amanitin on ribonucleic acid synthesis and on ribonucleic acid polymerase in mouse liver nuclei. Biochem J. 1967 Nov;105(2):779–782. doi: 10.1042/bj1050779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TRAUB A., KAUFMANN E., GINZBURG-TIETZ Y. STUDIES ON NUCLEAR RIBOSOMES. I. ASSOCIATION OF DPN-PYROPHOSPHORYLASE WITH NUCLEAR RIBOSOMES IN NORMAL AND NEOPLASTIC TISSUES. Exp Cell Res. 1964 Apr;34:371–383. doi: 10.1016/0014-4827(64)90372-6. [DOI] [PubMed] [Google Scholar]
- Widnell C. C., Tata J. R. A procedure for the isolation of enzymically active rat-liver nuclei. Biochem J. 1964 Aug;92(2):313–317. doi: 10.1042/bj0920313. [DOI] [PMC free article] [PubMed] [Google Scholar]


