Abstract
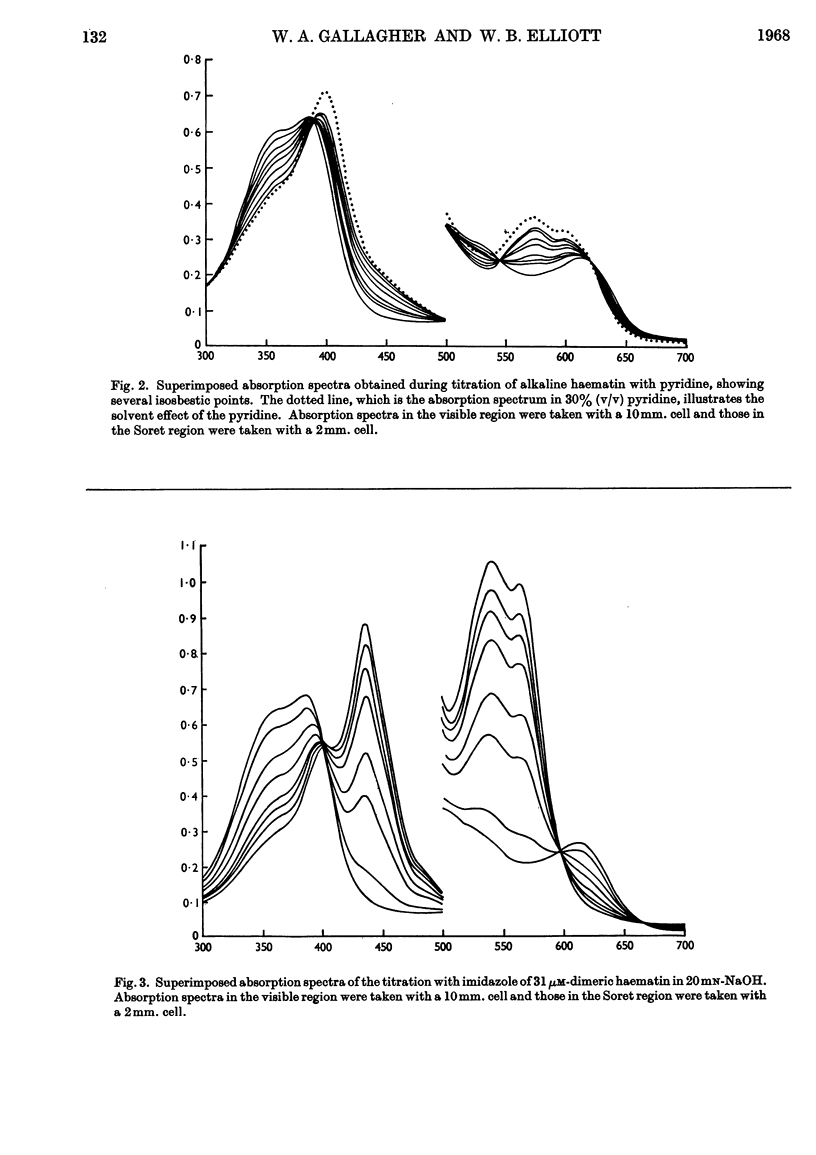
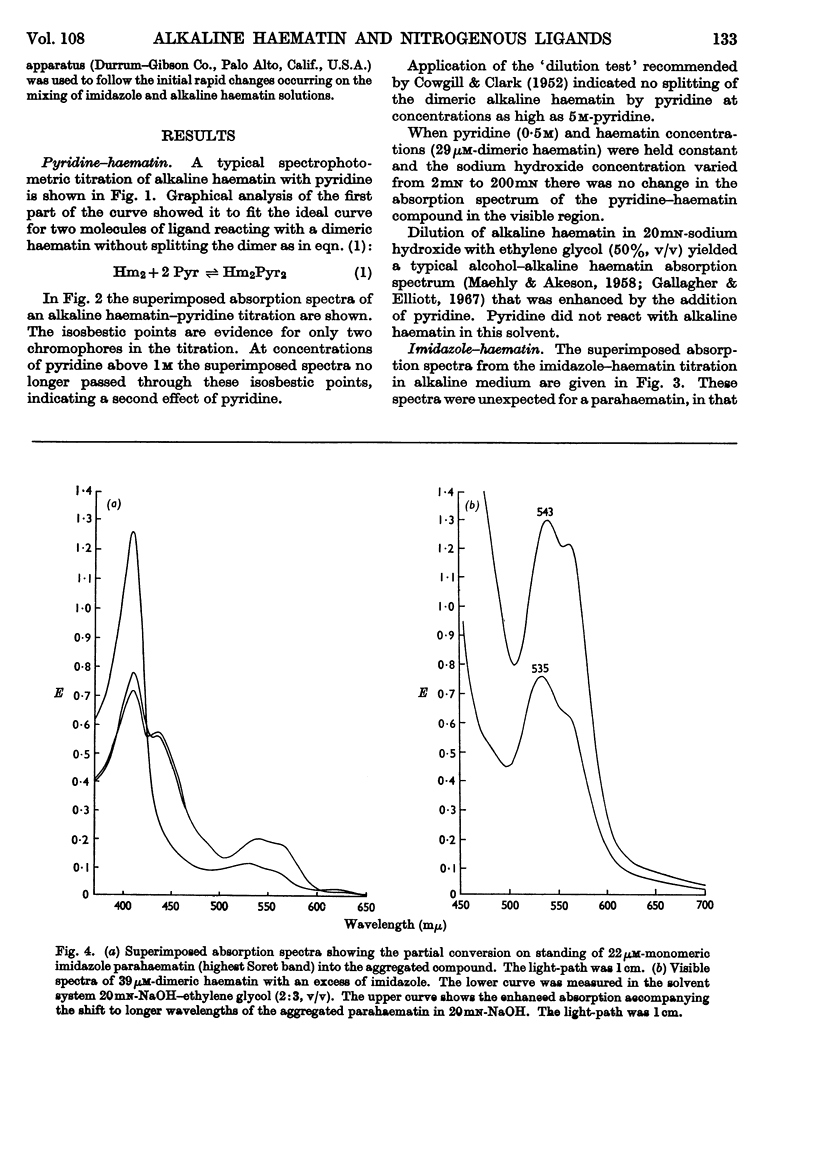
1. Pyridine reacts with alkaline haematin to form a dipyridine dihydroxy dimeric haematin in which there is no competition between pyridine and OH− for coordination positions on the iron of haematin. 2. Imidazole competes directly with OH− to form the monomeric imidazole parahaematin in alkaline media. 3. The monomeric imidazole parahaematin aggregates readily to form a species that is precipitated on standing. 4. A structure is proposed for the haematin–pyridine compound in alkaline media.
Full text
PDF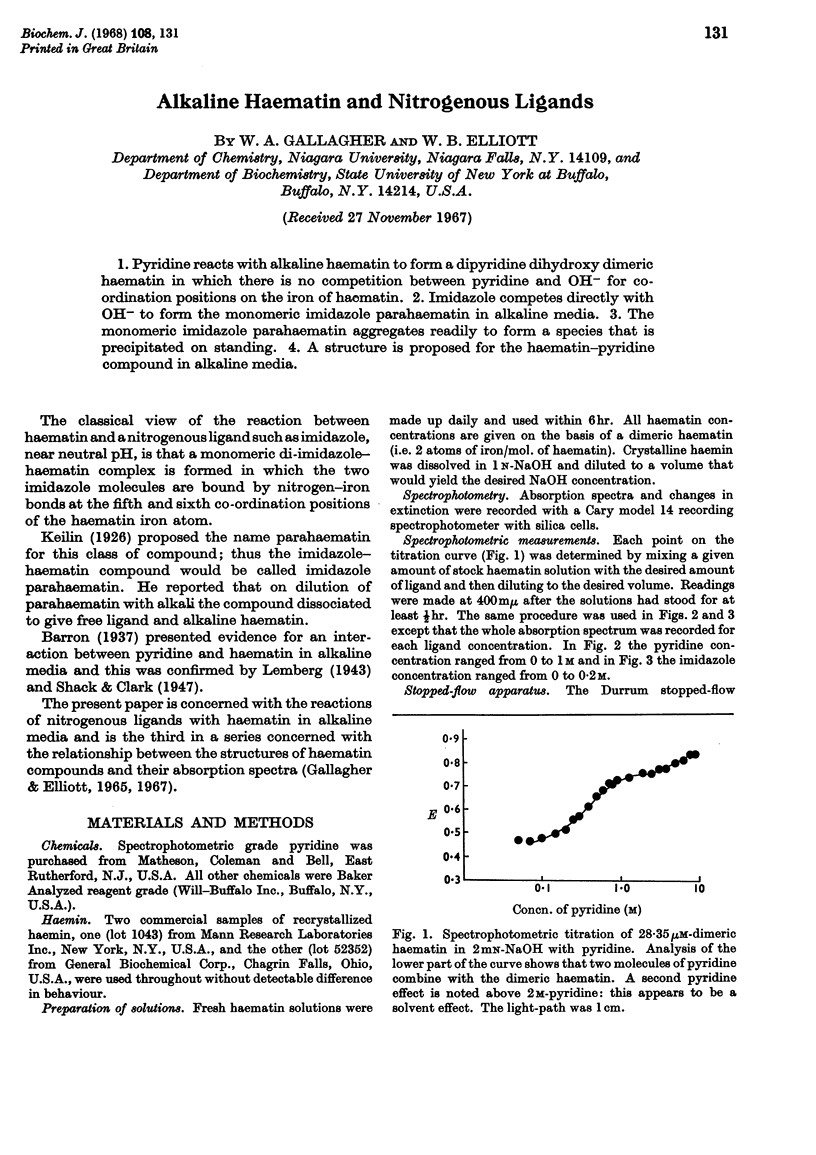


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- COWGILL R. W., CLARK W. M. Metalloporphyrins. VII. Coordination of imidazoles with ferrimesoporphyrin. J Biol Chem. 1952 Sep;198(1):33–61. [PubMed] [Google Scholar]
- Gallagher W. A., Elliott W. B. Caffeine derivatives of haematin compounds. Biochem J. 1967 Nov;105(2):461–465. doi: 10.1042/bj1050461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher W. A., Elliott W. B. The formation of pyridine haemochromogen. Biochem J. 1965 Oct;97(1):187–193. doi: 10.1042/bj0970187. [DOI] [PMC free article] [PubMed] [Google Scholar]