Abstract
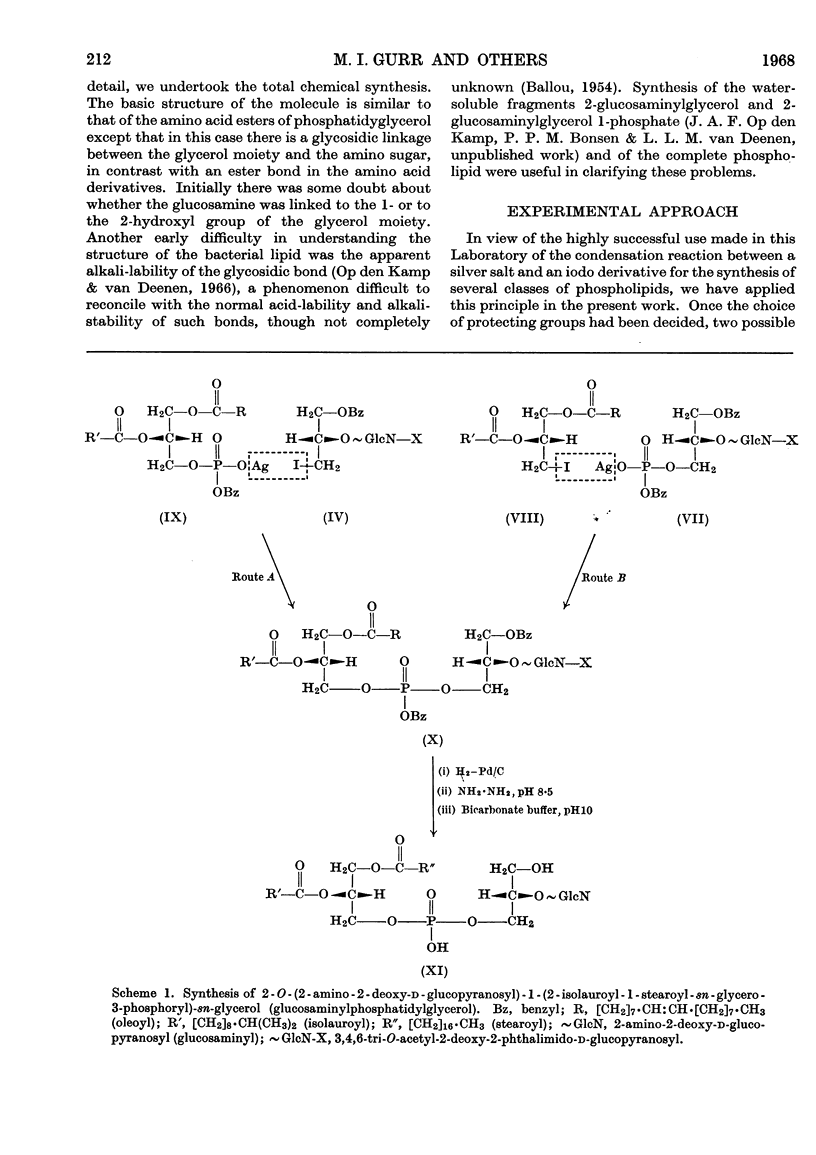
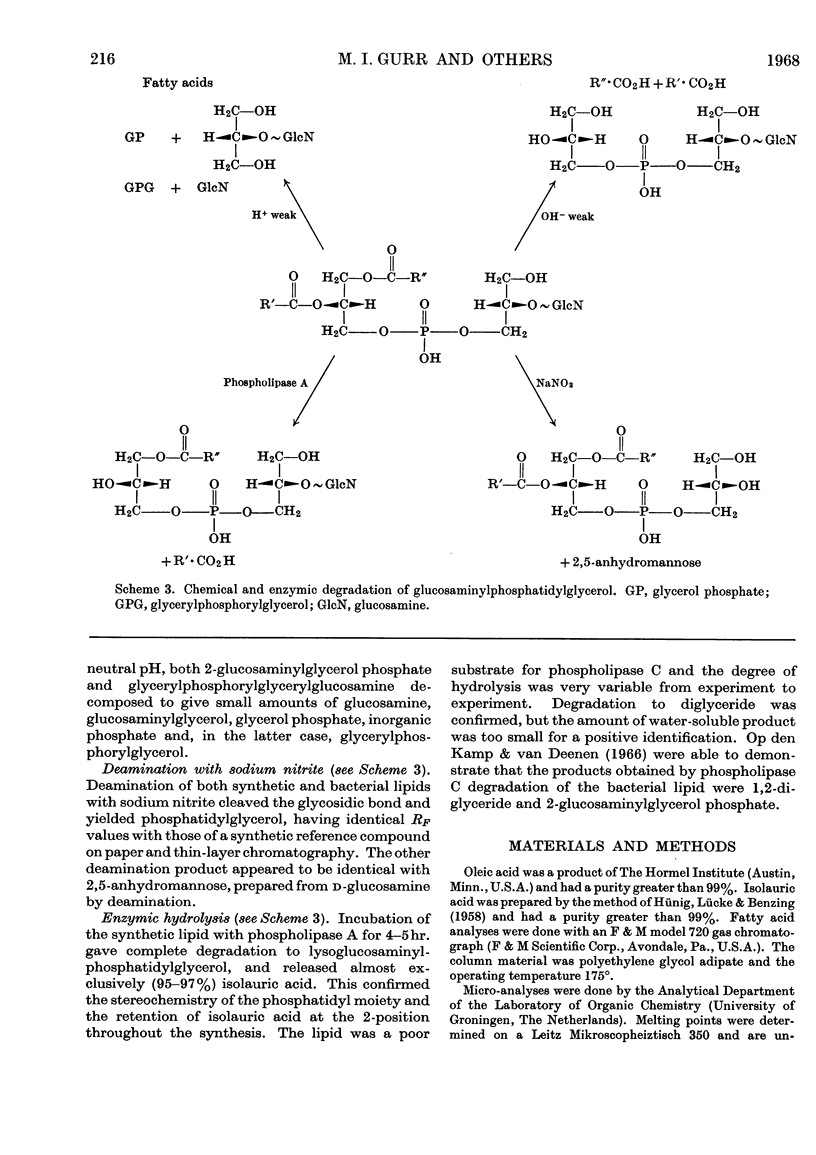
1. We describe the synthesis of a glucosamine derivative of phosphatidylglycerol having the same structure as that of the natural compound isolated from Bacillus megaterium. 2. 2-O-(3,4,6-Tri-O-acetyl-2-deoxy-2-phthalimido-d-glucopyranosyl)-3-O-benzyl-1-iodo-sn-glycerol was prepared by a Königs–Knorr condensation between 3-O-benzyl-1-toluene-p-sulphonyl-sn-glycerol and 3,4,6-tri-O-acetyl-1-bromo-2-deoxy-2-phthalimido-d-glucopyranose followed by replacement of the toluene-p-sulphonyl group with iodine. The iodide was treated with the silver salt of 2-isolauroyl-1-oleoyl-sn-glycerol 3-(monobenzyl hydrogen phosphate) to form the fully protected phosphoglycolipid. 3. Removal of benzyl protecting groups by catalytic hydrogenolysis, phthaloyl group with hydrazine and acetyl groups with pH10 buffer furnished 2-O-(2-amino-2-deoxy-d-glucopyranosyl)-1-(2-isolauroyl-1-stearoyl-sn-glycero-3-phosphoryl)-sn-glycerol. 4. The synthetic and natural compounds appeared identical when compared by chromatography and by identification of hydrolysis products from chemical and enzymic degradations.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BADDILEY J., BUCHANAN J. G., RAJBHANDARY U. L., SANDERSON A. R. Teichoic acid from the walls of Staphylococcus aureus H. Structure of the N-acetylglucosaminyl-ribitol residues. Biochem J. 1962 Mar;82:439–448. doi: 10.1042/bj0820439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BALLOU C. E. Alkali-sensitive glycosides. Adv Carbohydr Chem. 1954;9:59–95. doi: 10.1016/s0096-5332(08)60372-0. [DOI] [PubMed] [Google Scholar]
- Baer E., Rao K. V. Lipoamino acid. Synthesis of amino acid esters of phosphatidyl glycerols. Can J Biochem. 1966 Jun;44(6):899–915. doi: 10.1139/o66-107. [DOI] [PubMed] [Google Scholar]
- Bonsen P. P., de Haas G. H., van Deenen L. L. Synthesis and enzymic hydrolysis of an O-alanyl ester of phosphatidyl glycerol. Biochim Biophys Acta. 1965 Jul 7;106(1):93–105. doi: 10.1016/0005-2760(65)90098-6. [DOI] [PubMed] [Google Scholar]
- Bonsen P. P., de Haas G. H., van Deenen L. L. Synthetic and structural investigations on 3-phosphatidyl-1'-(3'-O-L-lysyl)glycerol. Biochemistry. 1967 Apr;6(4):1114–1120. doi: 10.1021/bi00856a021. [DOI] [PubMed] [Google Scholar]
- DAWSON R. M. A hydrolytic procedure for the identification and estimation of individual phospholipids in biological samples. Biochem J. 1960 Apr;75:45–53. doi: 10.1042/bj0750045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GALE E. F., FOLKES J. P. THE INCORPORATION OF GLYCEROL AND LYSINE INTO THE LIPID FRACTION OF STAPHYLOCOCCUS AUREUS. Biochem J. 1965 Feb;94:390–400. doi: 10.1042/bj0940390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOUTSMULLER U. M., van DEENEN L. Identification of a bacterial phospholipid as an O-ornithine ester of phosphatidyl glycerol. Biochim Biophys Acta. 1963 Apr 23;70:211–213. doi: 10.1016/0006-3002(63)90743-1. [DOI] [PubMed] [Google Scholar]
- Houtsmuller U. M., van Deenen L. L. On the amino acid esters of phosphatidyl glycerol from bacteria. Biochim Biophys Acta. 1965 Dec 2;106(3):564–576. doi: 10.1016/0005-2760(65)90072-x. [DOI] [PubMed] [Google Scholar]
- Lennarz W. J., Bonsen P. P., van Deenen L. L. Substrate specificity of O-L-lysylphosphatidylglycerol synthetase. Enzymatic studies on the structure of O-L-lysylphosphatidylglycerol. Biochemistry. 1967 Aug;6(8):2307–2312. doi: 10.1021/bi00860a005. [DOI] [PubMed] [Google Scholar]
- Lennarz W. J., Nesbitt J. A., 3rd, Reiss J. The participation of sRNA in the enzymatic synthesis of O-L-lysyl phosphatidylgylcerol in Staphylococcus aureus. Proc Natl Acad Sci U S A. 1966 Apr;55(4):934–941. doi: 10.1073/pnas.55.4.934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TREVELYAN W. E., PROCTER D. P., HARRISON J. S. Detection of sugars on paper chromatograms. Nature. 1950 Sep 9;166(4219):444–445. doi: 10.1038/166444b0. [DOI] [PubMed] [Google Scholar]
- den Kamp JA O. P., Houtsmuller U. M., van Deenen L. L. On the phospholipids of Bacillus megaterium. Biochim Biophys Acta. 1965 Oct 4;106(2):438–441. doi: 10.1016/0005-2760(65)90059-7. [DOI] [PubMed] [Google Scholar]


