Abstract
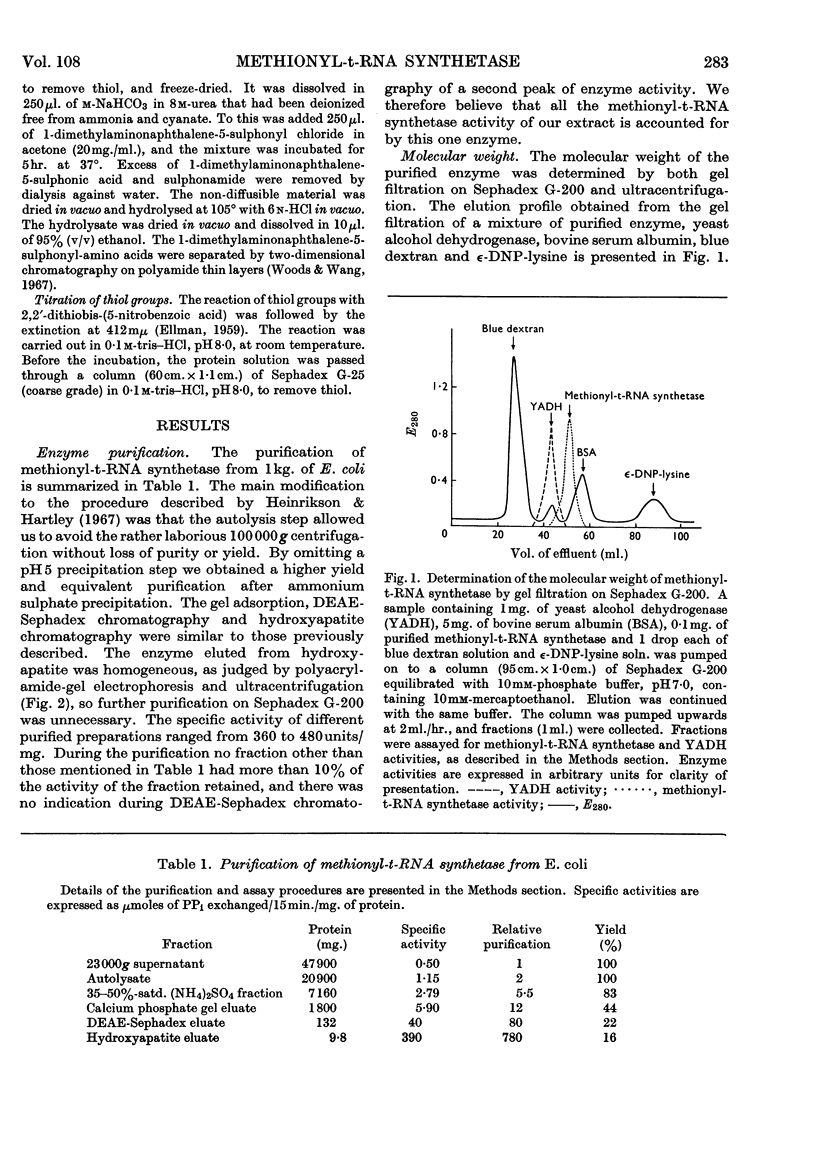
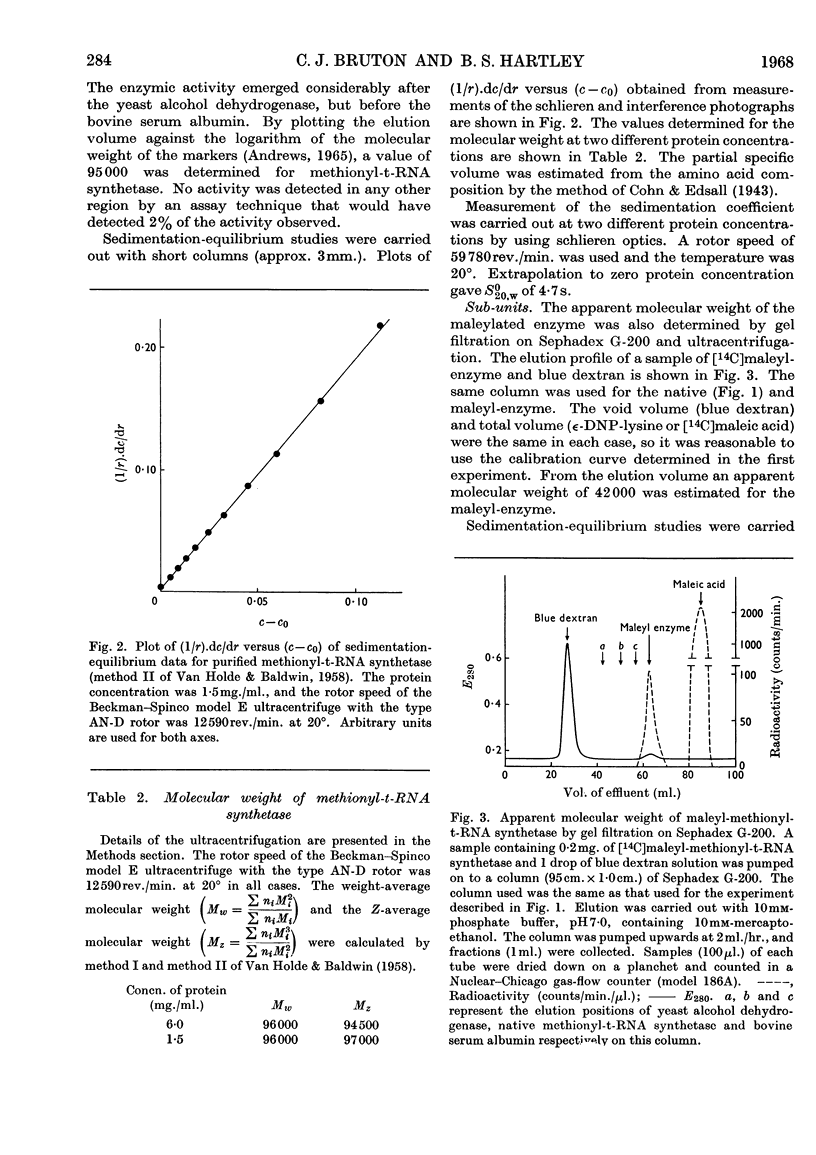
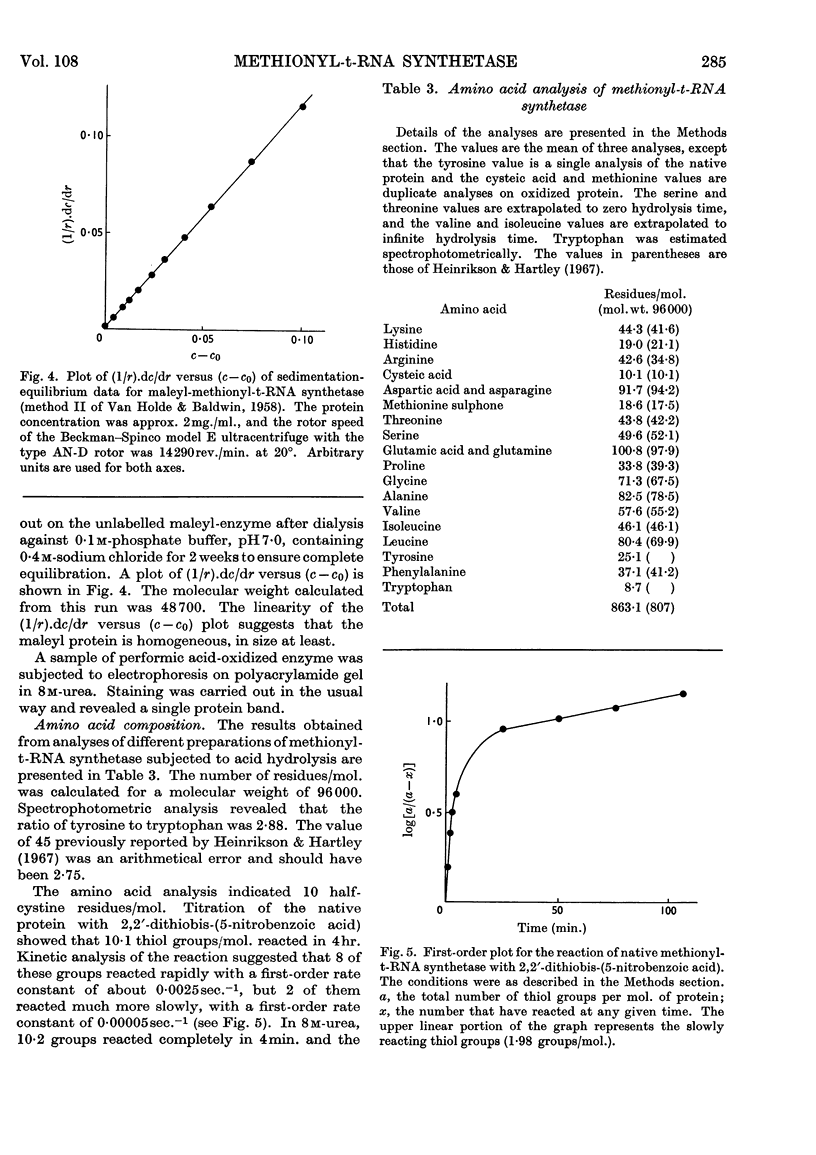
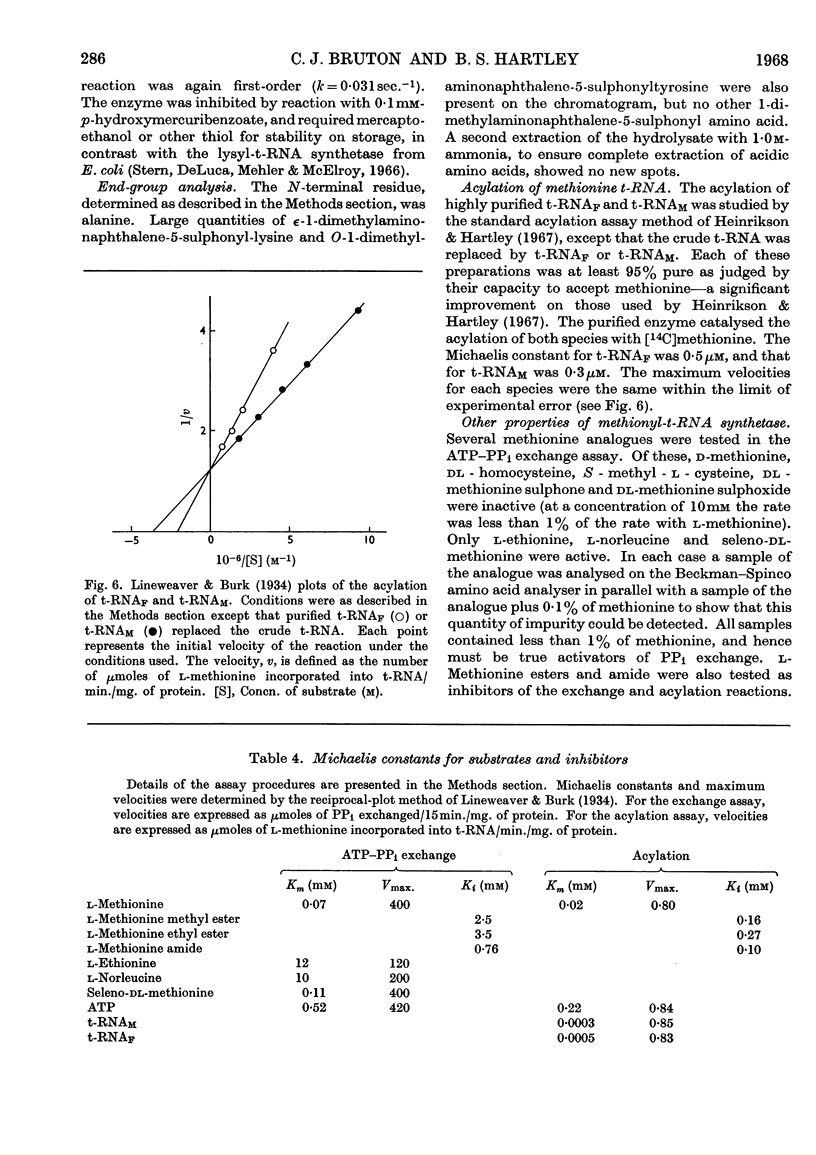
1. The purification of methionyl-transfer-RNA synthetase from Escherichia coli by a modified technique gives a 16% yield of a protein that appears homogeneous by the criteria of disc gel electrophoresis, ultracentrifugation and end-group analysis. 2. The molecular weight is 96000 and the protein consists of two sub-units of 48000, which appear to be identical. The amino acid composition and thiol content are reported. 3. Kinetic data are reported for analogues of methionine and for pure t-RNAF and t-RNAM, which are respectively the methionine transfer RNA that can exist in the formylmethionyl form and the one that can exist only in the methionyl form. The enzyme binds and acylates both species of transfer RNA identically.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams J. M., Capecchi M. R. N-formylmethionyl-sRNA as the initiator of protein synthesis. Proc Natl Acad Sci U S A. 1966 Jan;55(1):147–155. doi: 10.1073/pnas.55.1.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson J. S., Bretscher M. S., Clark B. F., Marcker K. A. A GTP requirement for binding initiator tRNA to ribosomes. Nature. 1967 Jul 29;215(5100):490–492. doi: 10.1038/215490a0. [DOI] [PubMed] [Google Scholar]
- Andrews P. The gel-filtration behaviour of proteins related to their molecular weights over a wide range. Biochem J. 1965 Sep;96(3):595–606. doi: 10.1042/bj0960595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BEAVEN G. H., HOLIDAY E. R. Ultraviolet absorption spectra of proteins and amino acids. Adv Protein Chem. 1952;7:319–386. doi: 10.1016/s0065-3233(08)60022-4. [DOI] [PubMed] [Google Scholar]
- Baldwin A. N., Berg P. Purification and properties of isoleucyl ribonucleic acid synthetase from Escherichia coli. J Biol Chem. 1966 Feb 25;241(4):831–838. [PubMed] [Google Scholar]
- Bretscher M. S., Marcker K. A. Polypeptidyl-sigma-ribonucleic acid and amino-acyl-sigma-ribonucleic acid binding sites on ribosomes. Nature. 1966 Jul 23;211(5047):380–384. doi: 10.1038/211380a0. [DOI] [PubMed] [Google Scholar]
- Butler P. J., Harris J. I., Hartley B. S., Leberman R. Reversible blocking of peptide amino groups by maleic anhydride. Biochem J. 1967 Jun;103(3):78P–79P. [PMC free article] [PubMed] [Google Scholar]
- CHAPEVILLE F., LIPMANN F., VON EHRENSTEIN G., WEISBLUM B., RAY W. J., Jr, BENZER S. On the role of soluble ribonucleic acid in coding for amino acids. Proc Natl Acad Sci U S A. 1962 Jun 15;48:1086–1092. doi: 10.1073/pnas.48.6.1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CRESTFIELD A. M., STEIN W. H., MOORE S. On the preparation of bovine pancreatic ribonuclease A. J Biol Chem. 1963 Feb;238:618–621. [PubMed] [Google Scholar]
- Calendar R., Berg P. Purification and physical characterization of tyrosyl ribonucleic acid synthetases from Escherichia coli and Bacillus subtilis. Biochemistry. 1966 May;5(5):1681–1690. doi: 10.1021/bi00869a033. [DOI] [PubMed] [Google Scholar]
- Clark B. F., Marcker K. A. The role of N-formyl-methionyl-sRNA in protein biosynthesis. J Mol Biol. 1966 Jun;17(2):394–406. doi: 10.1016/s0022-2836(66)80150-x. [DOI] [PubMed] [Google Scholar]
- Clark F. C., Marcker K. A. Coding response of N-fromyl-methionyl-sRNA to UUG. Nature. 1965 Sep 4;207(5001):1038–1039. doi: 10.1038/2071038b0. [DOI] [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- ELLMAN G. L. Tissue sulfhydryl groups. Arch Biochem Biophys. 1959 May;82(1):70–77. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- GLENN J. L. Activation of L-methionine and L-ethionine by pH 5 fraction of rat liver. Arch Biochem Biophys. 1961 Oct;95:14–18. doi: 10.1016/0003-9861(61)90102-3. [DOI] [PubMed] [Google Scholar]
- Ghosh H. P., Khorana H. G. Studies on polynucleotides, LXXXIV. On the role of ribosomal subunits in protein synthesis. Proc Natl Acad Sci U S A. 1967 Dec;58(6):2455–2461. doi: 10.1073/pnas.58.6.2455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HABEEB A. F., CASSIDY H. G., SINGER S. J. Molecular structural effects produced in proteins by reaction with succinic anhydride. Biochim Biophys Acta. 1958 Sep;29(3):587–593. doi: 10.1016/0006-3002(58)90016-7. [DOI] [PubMed] [Google Scholar]
- HIRS C. H. The oxidation of ribonuclease with performic acid. J Biol Chem. 1956 Apr;219(2):611–621. [PubMed] [Google Scholar]
- HJERTEN S., LEVIN O., TISELIUS A. Protein chromatography on calcium phosphate columns. Arch Biochem Biophys. 1956 Nov;65(1):132–155. doi: 10.1016/0003-9861(56)90183-7. [DOI] [PubMed] [Google Scholar]
- Heinrikson R. L., Hartley B. S. Purification and properties of methionyl-transfer-ribonucleic acid synthetase from Escherichia coli. Biochem J. 1967 Oct;105(1):17–24. doi: 10.1042/bj1050017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KLOTZ I. M., KERESZTES-NAGY S. HEMERYTHRIN: MOLECULAR WEIGHT AND DISSOCIATION INTO SUBUNITS. Biochemistry. 1963 May-Jun;2:445–452. doi: 10.1021/bi00903a008. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Mitra S. K., Mehler A. H. The arginyl transfer ribonucleic acid synthetase of Escherichia coli. J Biol Chem. 1967 Dec 10;242(23):5490–5494. [PubMed] [Google Scholar]
- Nass G., Stöffler G. Molecular weight distribution of the aminoacyl-tRNA-synthetases of Escherichia coli by gel filtration. Mol Gen Genet. 1967;100(4):378–382. doi: 10.1007/BF00334065. [DOI] [PubMed] [Google Scholar]
- Stern R., DeLuca M., Mehler A. H., McElroy W. D. Role of sulfhydryl groups in activatin enzymes. Properties of Escherichia coli lysine-transfer ribonucleic acid synthetase. Biochemistry. 1966 Jan;5(1):126–130. [PubMed] [Google Scholar]
- Stern R., Mehler A. H. Lysyl-sRNA synthetase from Escherichia coli. Biochem Z. 1965 Aug 19;342(4):400–409. [PubMed] [Google Scholar]
- Stulberg M. P. The isolation and properties of phenylalanyl ribonucleic acid synthetase from Escherichia coli B. J Biol Chem. 1967 Mar 10;242(5):1060–1064. [PubMed] [Google Scholar]
- WALLER J. P. THE NH2-TERMINAL RESIDUES OF THE PROTEINS FROM CELL-FREE EXTRACTS OF E. COLI. J Mol Biol. 1963 Nov;7:483–496. doi: 10.1016/s0022-2836(63)80096-0. [DOI] [PubMed] [Google Scholar]
- Webster R. E., Engelhardt D. L., Zinder N. D. In vitro protein synthesis: chain initiation. Proc Natl Acad Sci U S A. 1966 Jan;55(1):155–161. doi: 10.1073/pnas.55.1.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods K. R., Wang K. T. Separation of dansyl-amino acids by polyamide layer chromatography. Biochim Biophys Acta. 1967 Feb 21;133(2):369–370. doi: 10.1016/0005-2795(67)90078-5. [DOI] [PubMed] [Google Scholar]


