Abstract
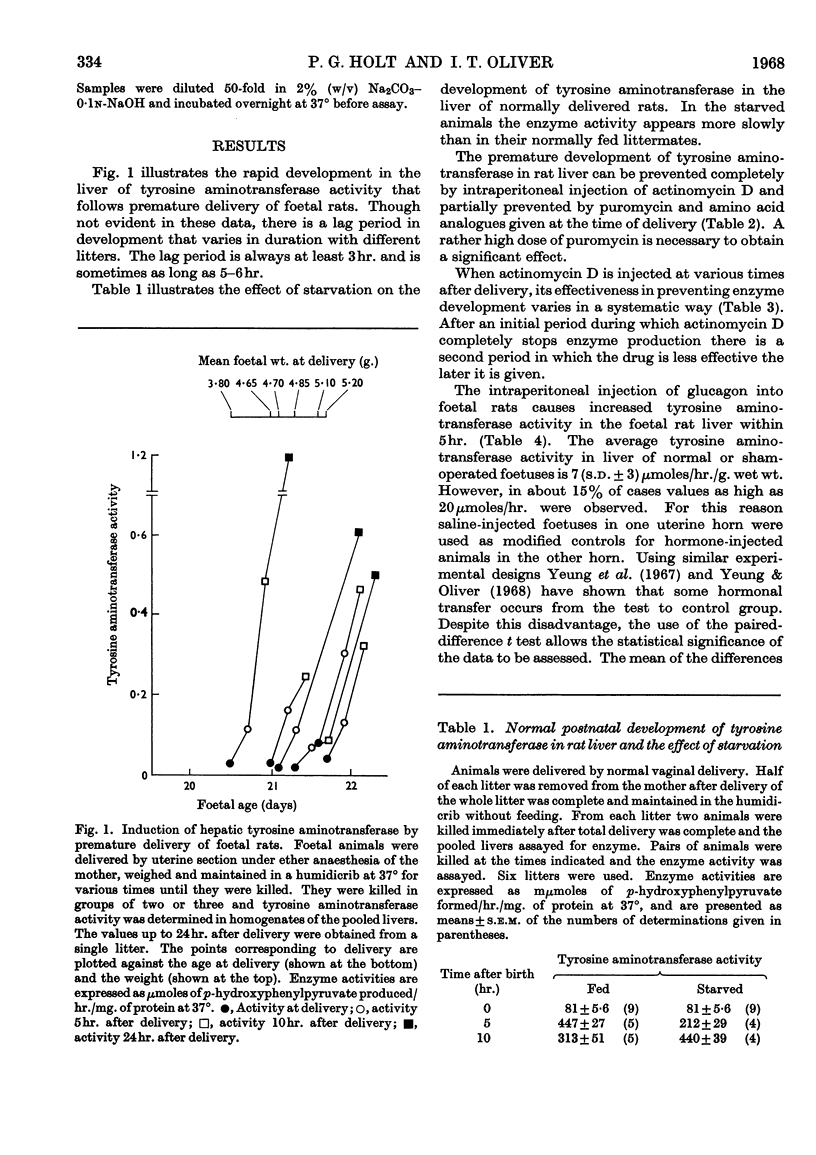
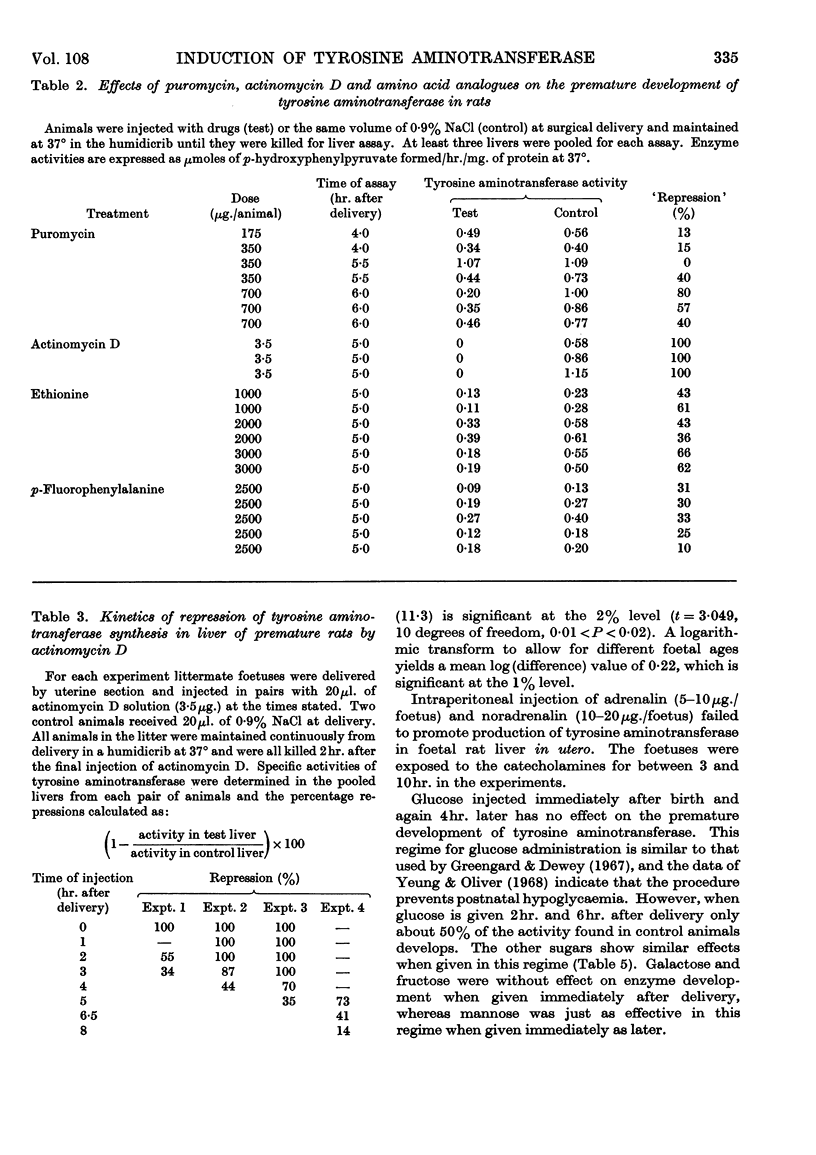
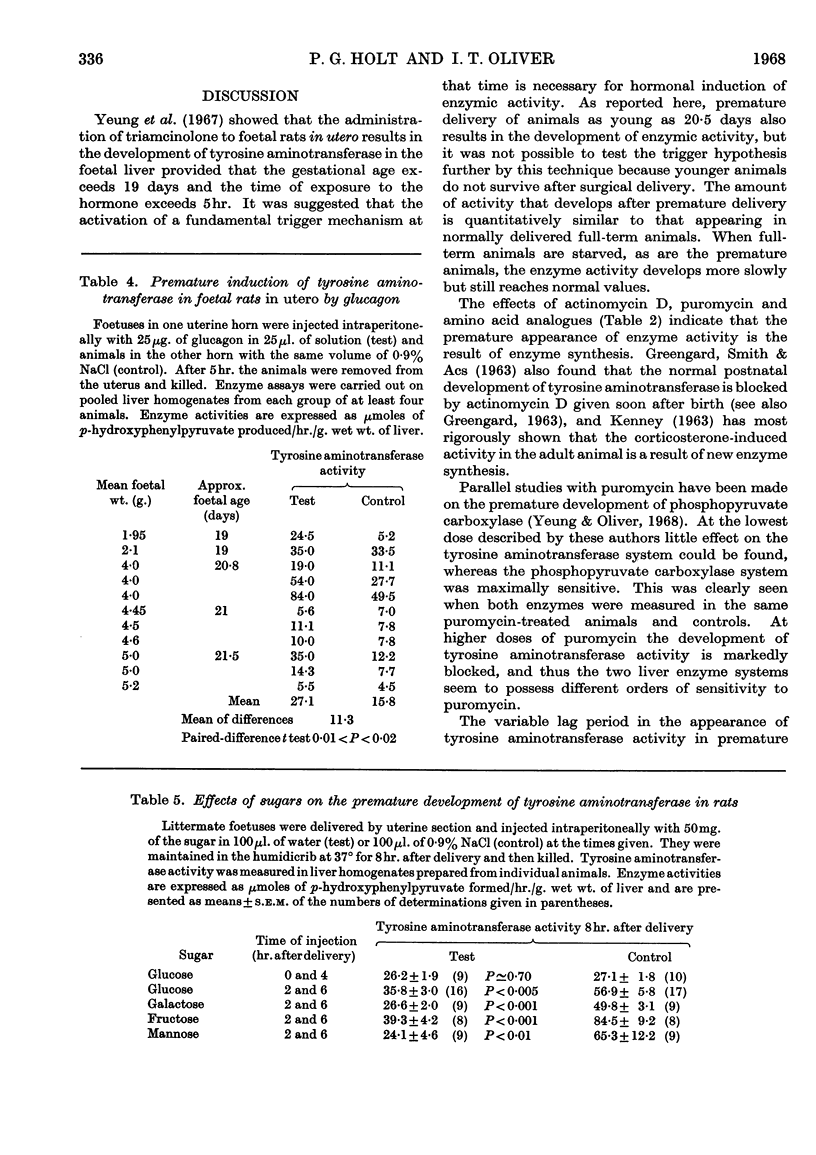
1. Premature delivery of foetal rats by uterine section results in the rapid appearance of tyrosine aminotransferase activity in foetal liver, after an initial lag period of 3–6hr. 2. The premature induction of activity is completely repressible by actinomycin D given soon after delivery and partially repressible by puromycin and amino acid analogues. 3. Glucagon injections into foetal rats in utero lead to production of tyrosine aminotransferase in the foetal liver, but adrenalin and nor-adrenalin are without effect. 4. Injections of glucose, galactose, fructose and mannose into prematurely delivered rats repress the development of tyrosine aminotransferase activity about 50% when they are given 2hr. after delivery, but glucose has no significant effect when injected at delivery. 5. The results are discussed in relation to current hypotheses on the role of hormones in enzyme induction in foetal development.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- GREENGARD O., SMITH M. A., ACS G. Relation of cortisone and synthesis of ribonucleic acid to induced and developmental enzyme formation. J Biol Chem. 1963 Apr;238:1548–1551. [PubMed] [Google Scholar]
- Greengard O., Dewey H. K. Initiation by glucagon of the premature development of tyrosine aminotransferase, serine dehydratase, and glucose-6-phosphatase in fetal rat liver. J Biol Chem. 1967 Jun 25;242(12):2986–2991. [PubMed] [Google Scholar]
- HAREL L., HAREL J., BOER A., IMBENOTTE J., CARPENI N. PERSISTANCE D'UNE SYNTH'ESE DE D-RNA DANS LE FOIE DE RAT TRAIT'E PAR L'ACTINOMYCINE. Biochim Biophys Acta. 1964 Jun 22;87:212–218. [PubMed] [Google Scholar]
- Holt P. G., Oliver I. T. Plasma corticosterone concentrations in the perinatal rat. Biochem J. 1968 Jun;108(2):339–341. doi: 10.1042/bj1080339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KENNEY F. T. MECHANISM OF HORMONAL CONTROL OF RAT LIVER TYROSINE TRANSAMINASE. Adv Enzyme Regul. 1963;1:137–150. doi: 10.1016/0065-2571(63)90014-1. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Reich E., Goldberg I. H. Actinomycin and nucleic acid function. Prog Nucleic Acid Res Mol Biol. 1964;3:183–234. doi: 10.1016/s0079-6603(08)60742-4. [DOI] [PubMed] [Google Scholar]
- SERENI F., KENNEY F. T., KRETCHMER N. Factors influencing the development of tyrosine-alpha-ketoglutarate transaminase activity in rat liver. J Biol Chem. 1959 Mar;234(3):609–612. [PubMed] [Google Scholar]
- Tata J. R. The formation and distribution of ribosomes during hormone-induced growth and development. Biochem J. 1967 Jul;104(1):1–16. doi: 10.1042/bj1040001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeung D., Oliver I. T. Factors affecting the premature induction of phosphopyruvate carboxylase in neonatal rat liver. Biochem J. 1968 Jun;108(2):325–331. doi: 10.1042/bj1080325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeung D., Stanley R. S., Oliver I. T. Development of gluconeogenesis in neonatal rat liver. Effect of triamcinolone. Biochem J. 1967 Dec;105(3):1219–1227. doi: 10.1042/bj1051219. [DOI] [PMC free article] [PubMed] [Google Scholar]


