Abstract
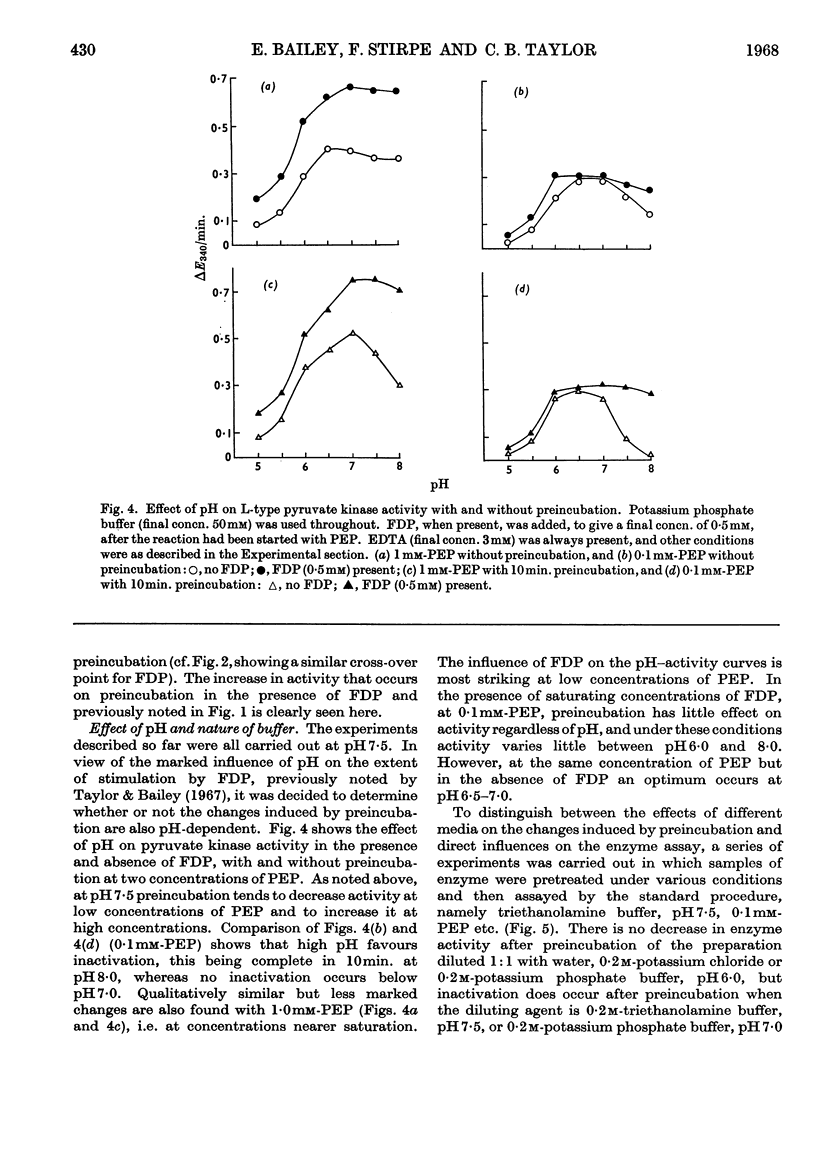
1. Preincubation of partially purified rat liver L-type pyruvate kinase at 25° for 10min. causes a marked increase in co-operativity with respect to both the substrate, phosphoenolpyruvate, and the allosteric activator, fructose 1,6-diphosphate. 2. The results are consistent with the existence of two forms of liver L-type pyruvate kinase, designated forms LA and LB. It is postulated that form LA has a low Km for phosphoenolpyruvate (about 0·1mm) and is not allosterically activated, whereas form LB is allosterically activated by fructose 1,6-diphosphate, exhibiting in the absence of the activator sigmoidal kinetics with half-maximal activity at about 1mm-phosphoenolpyruvate. In the presence of fructose 1,6-diphosphate, form LB gives Michaelis–Menten kinetics with Km less than 0·1mm. It is further postulated that preincubation converts form LA into form LB. 3. The influence of pH on the preincubation effect was studied. 4. The inhibition of pyruvate kinase by Cu2+ was studied in detail. Though phosphoenolpyruvate and fructose 1,6-diphosphate readily protect the enzyme against Cu2+ inhibition, little evidence of significant reversal of the inhibition by these compounds could be found. 5. The effects of starvation, fructose feeding and preincubation on the pyruvate kinase activity of crude homogenates of various tissues of the rat were also studied.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bailey E., Taylor C. B., Bartley W. Effect of dietary carbohydrates on hepatic lipogenesis in the rat. Nature. 1968 Feb 3;217(5127):471–472. doi: 10.1038/217471b0. [DOI] [PubMed] [Google Scholar]
- Hess B., Haeckel R., Brand K. FDP-activation of yeast pyruvate kinase. Biochem Biophys Res Commun. 1966 Sep 22;24(6):824–831. doi: 10.1016/0006-291x(66)90322-6. [DOI] [PubMed] [Google Scholar]
- MONOD J., CHANGEUX J. P., JACOB F. Allosteric proteins and cellular control systems. J Mol Biol. 1963 Apr;6:306–329. doi: 10.1016/s0022-2836(63)80091-1. [DOI] [PubMed] [Google Scholar]
- MONOD J., WYMAN J., CHANGEUX J. P. ON THE NATURE OF ALLOSTERIC TRANSITIONS: A PLAUSIBLE MODEL. J Mol Biol. 1965 May;12:88–118. doi: 10.1016/s0022-2836(65)80285-6. [DOI] [PubMed] [Google Scholar]
- Passeron S., Jiménez de Asua L., Carminatti H. Fructose 1,6-diphosphate, a reactivator of Cu++-inhibited pyruvate kinase from liver. Biochem Biophys Res Commun. 1967 Apr 7;27(1):33–38. doi: 10.1016/s0006-291x(67)80035-4. [DOI] [PubMed] [Google Scholar]
- SOLVONUK P. F., COLLIER H. B. The pyruvic phosphoferase of erythrocytes. I. Properties of the enzyme and its activity in erythrocytes of various species. Can J Biochem Physiol. 1955 Jan;33(1):38–45. [PubMed] [Google Scholar]
- Tanaka T., Harano Y., Morimura H., Mori R. Evidence for the presence of two types of pyruvate kinase in rat liver. Biochem Biophys Res Commun. 1965 Oct 8;21(1):55–60. doi: 10.1016/0006-291x(65)90425-0. [DOI] [PubMed] [Google Scholar]


