Abstract
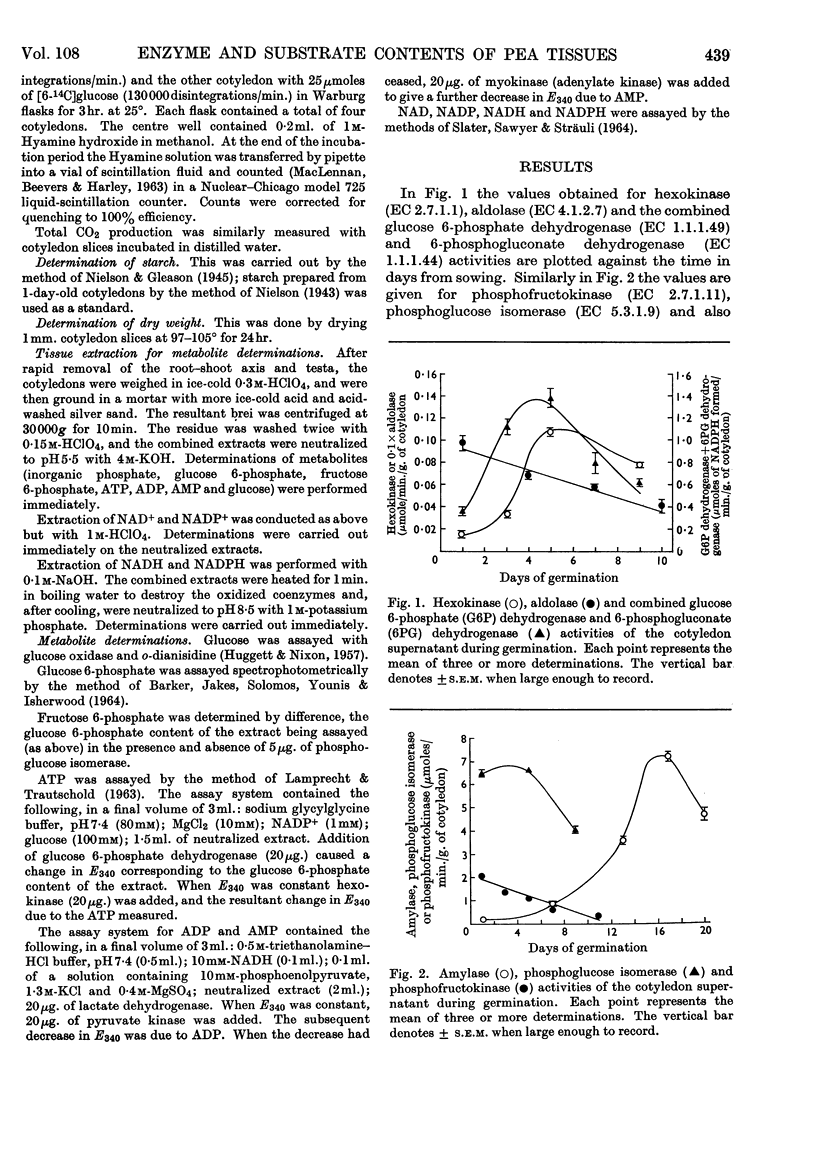
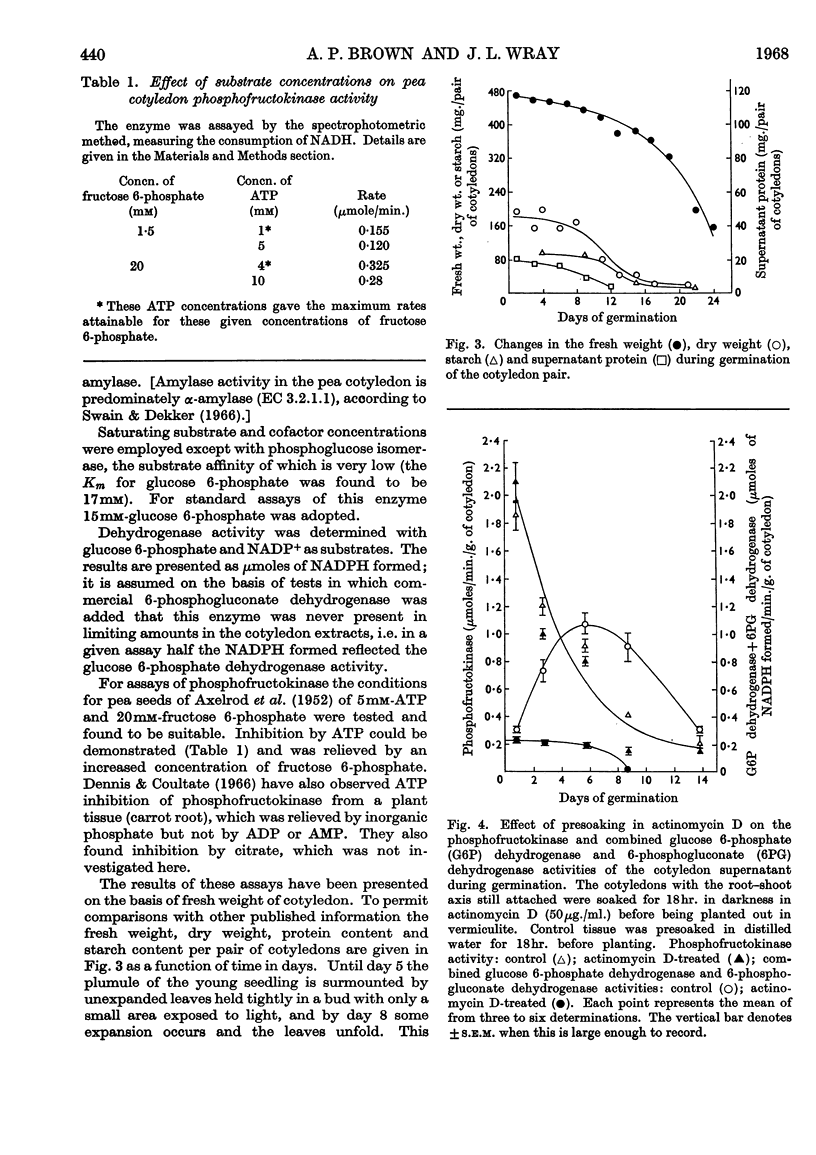
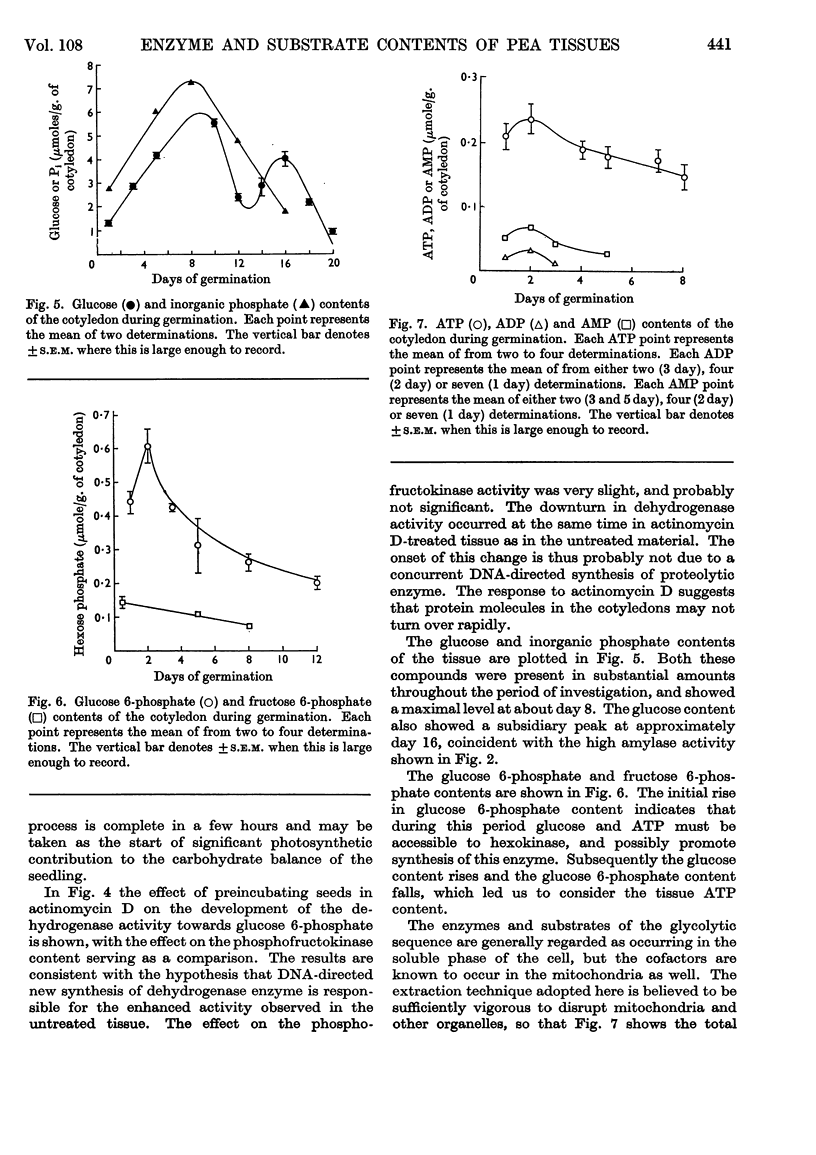
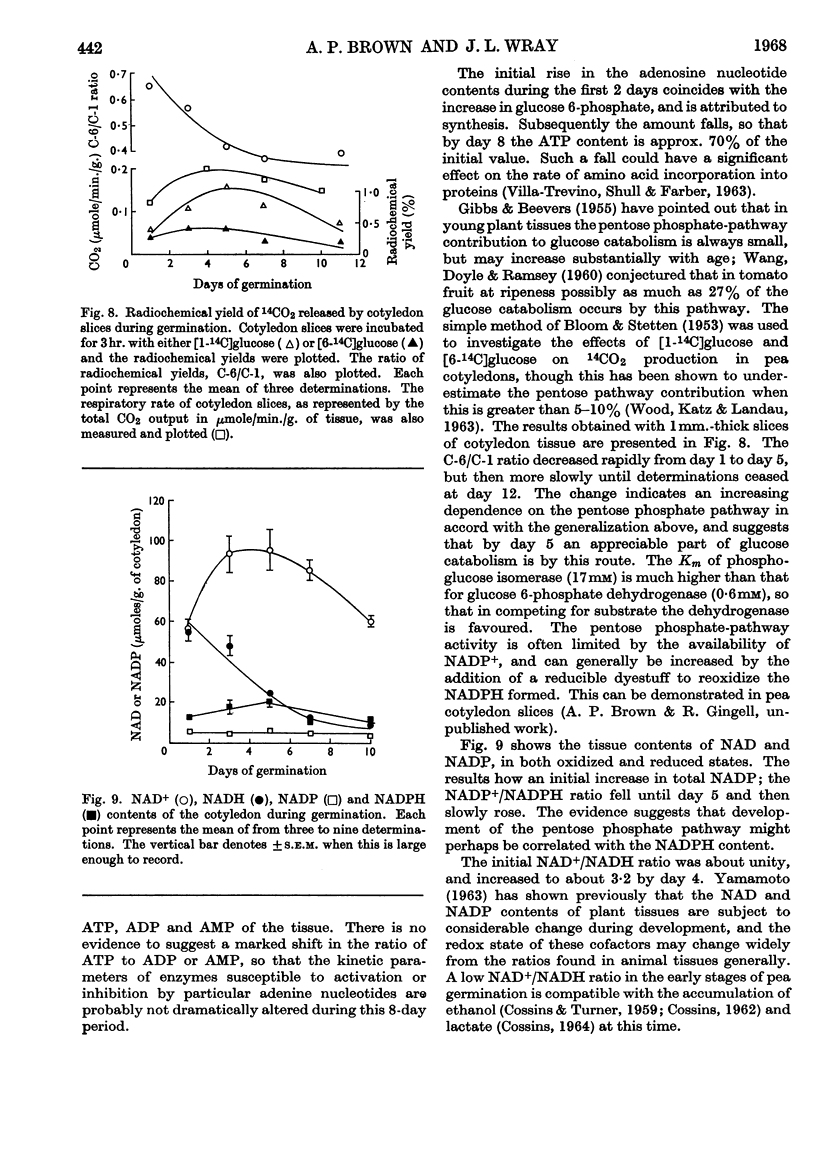
1. The activities of six enzymes (hexokinase, phosphoglucose isomerase, phosphofructokinase, aldolase, glucose 6-phosphate dehydrogenase and amylase) in extracts of pea cotyledons were determined. The activities during the first 10 days after germination showed individual and characteristic changes that indicate a specific control of both synthesis and destruction of enzymes. 2. Tissue contents of glucose, inorganic phosphate, glucose 6-phosphate, fructose 6-phosphate, ATP, ADP, AMP, NAD and NADP were also determined, and a correlation is reported between the substrate concentrations at day 1 and the subsequent enzymic activity. 3. The initial NAD+/NADH ratio value of 1 changed to about 3 by day 4; the NADP content was lower and changes in the oxidation state were less striking. The ratio of ATP to ADP and AMP remained virtually constant.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AXELROD B., SALTMAN P., BANDURSKI R. S., BAKER R. S. Phosphonexokinase in higher plants. J Biol Chem. 1952 May;197(1):89–96. [PubMed] [Google Scholar]
- DIPIETRO D. L., WEINHOUSE S. Hepatic glucokinase in the fed, fasted, and alloxan-diabetic rat. J Biol Chem. 1960 Sep;235:2542–2545. [PubMed] [Google Scholar]
- Dennis D. T., Coultate T. P. Phosphofructokinase, a regulatory enzyme in plants. Biochem Biophys Res Commun. 1966 Oct 20;25(2):187–191. doi: 10.1016/0006-291x(66)90578-x. [DOI] [PubMed] [Google Scholar]
- GLASER L., BROWN D. H. Purification and properties of d-glucose-6-phosphate dehydrogenase. J Biol Chem. 1955 Sep;216(1):67–79. [PubMed] [Google Scholar]
- Gibbs M., Beevers H. Glucose Dissimilation in the Higher Plant. Effect of Age of Tissue. Plant Physiol. 1955 Jul;30(4):343–347. doi: 10.1104/pp.30.4.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson M. K. Studies on glutathione S-alkyltransferase of the rat. Biochem J. 1966 Jan;98(1):44–56. doi: 10.1042/bj0980044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MANDELSTAM J. The intracellular turnover of protein and nucleic acids and its role in biochemical differentiation. Bacteriol Rev. 1960 Sep;24(3):289–308. doi: 10.1128/br.24.3.289-308.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maclennan D. H., Beevers H., Harley J. L. 'Compartmentation' of acids in plant tissues. Biochem J. 1963 Nov;89(2):316–327. doi: 10.1042/bj0890316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SALTMAN P. Hexokinase in higher plants. J Biol Chem. 1953 Jan;200(1):145–154. [PubMed] [Google Scholar]
- Schimke R. T., Sweeney E. W., Berlin C. M. Studies of the stability in vivo and in vitro of rat liver tryptophan pyrrolase. J Biol Chem. 1965 Dec;240(12):4609–4620. [PubMed] [Google Scholar]
- Slater T. F., Sawyer B., Sträuli U. An assay procedure for nicotinamide-adenine dinucleotides in rat liver and other tissues. Arch Int Physiol Biochim. 1964 Jun;72(3):427–447. doi: 10.3109/13813456409065351. [DOI] [PubMed] [Google Scholar]
- Swain R. R., Dekker E. E. Seed germination studies. II. Pathways for starch degradation in germinating pea seedlings. Biochim Biophys Acta. 1966 Jul 6;122(1):87–100. doi: 10.1016/0926-6593(66)90093-2. [DOI] [PubMed] [Google Scholar]
- VILLA-TREVINO S., SHULL K. H., FARBER E. The role of adenosine triphosphate deficiency in ethionine-induced inhibition of protein synthesis. J Biol Chem. 1963 May;238:1757–1763. [PubMed] [Google Scholar]


