Abstract
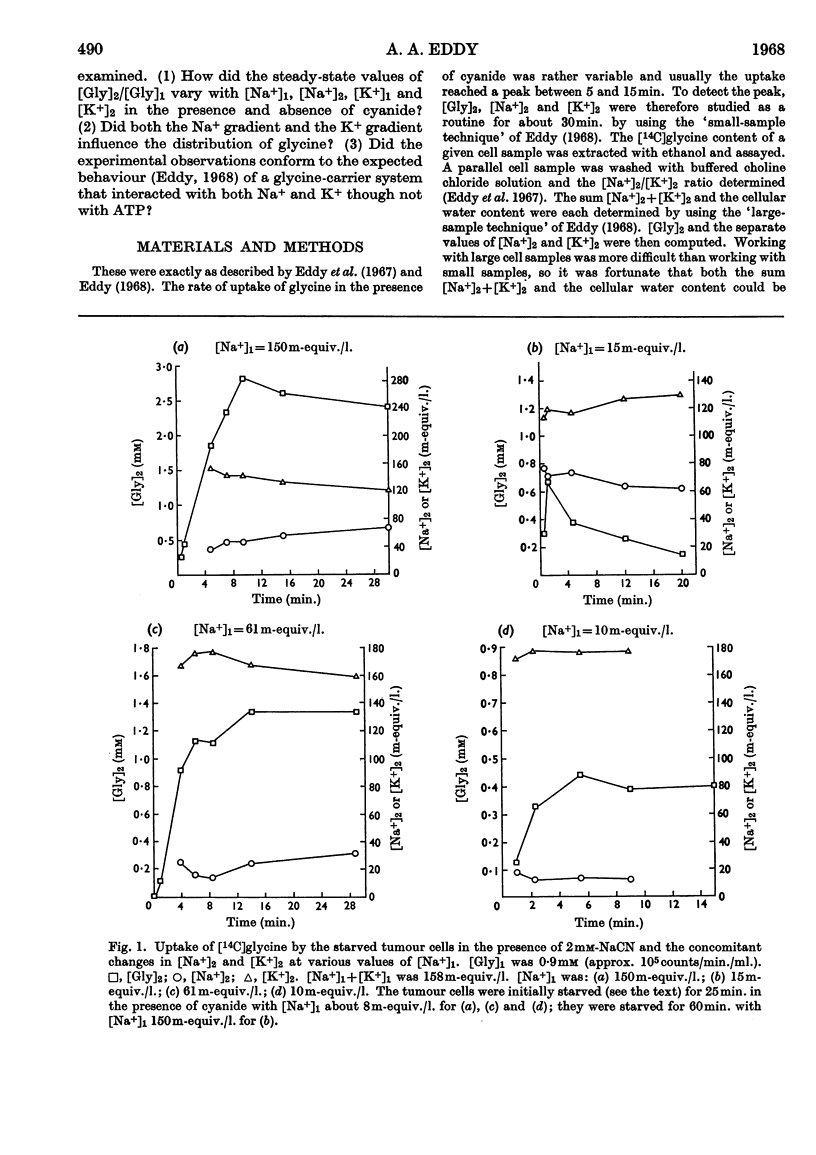
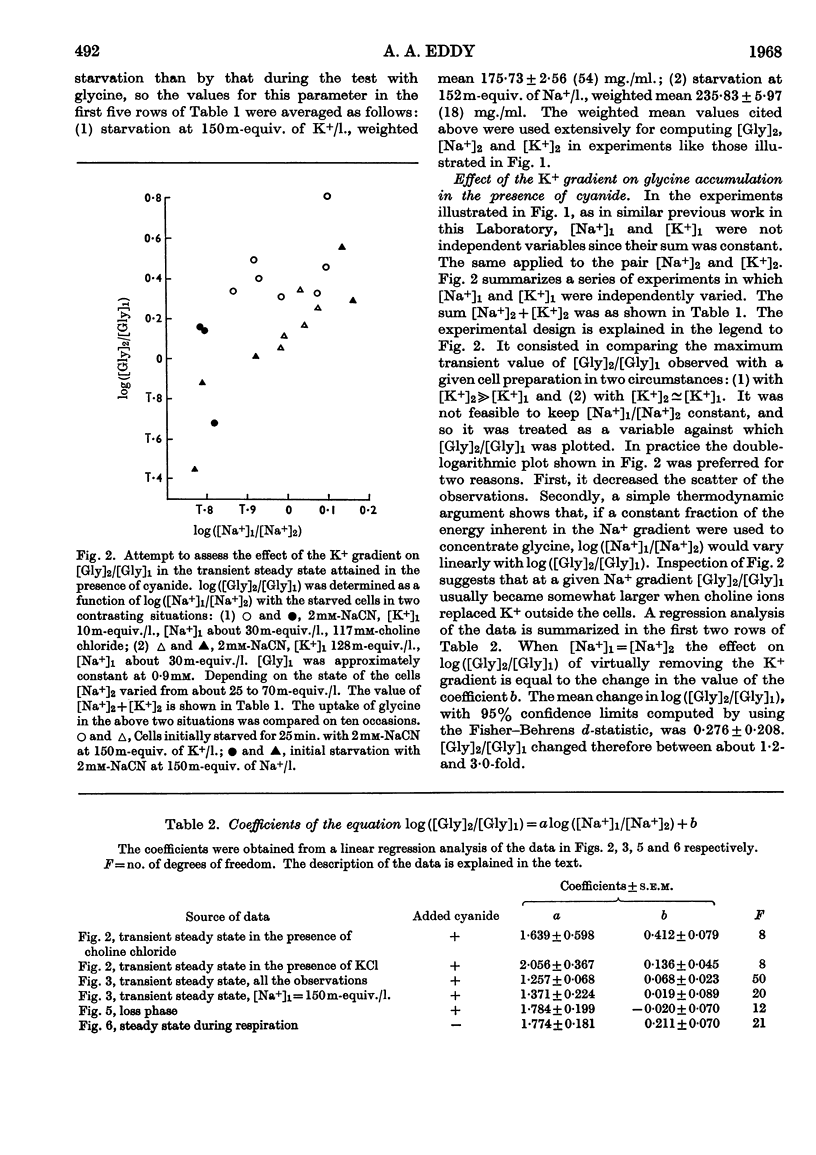
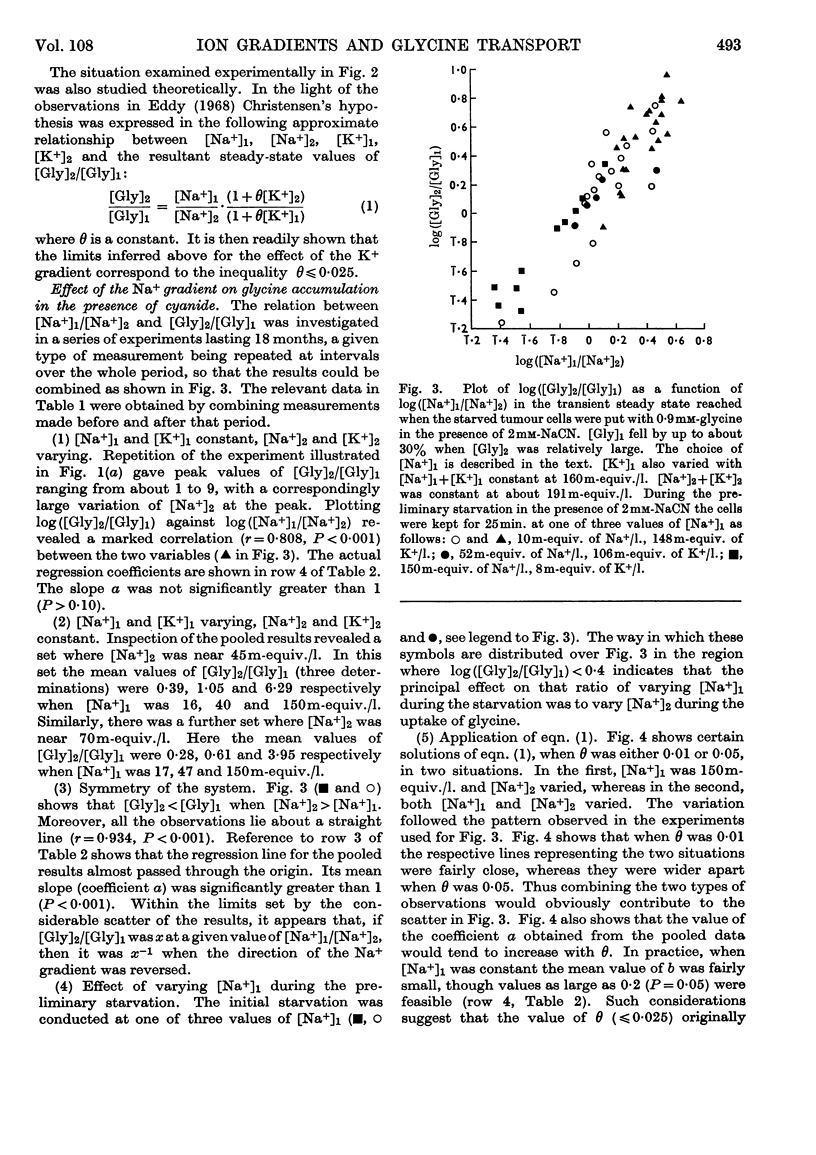
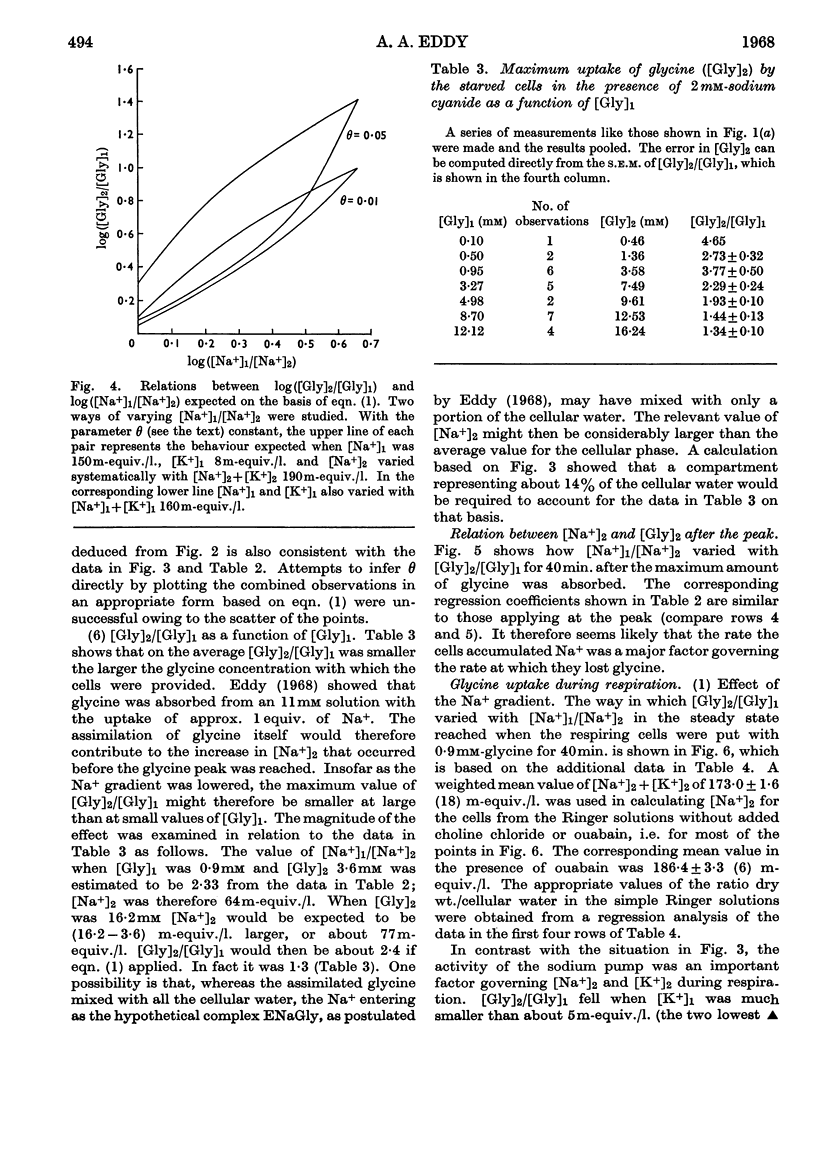
1. Tumour cells were starved to deplete them of ATP and transferred to 0·9mm-glycine in Ringer solutions containing 2mm-sodium cyanide and various Na+ and K+ concentrations. The uptake of glycine then usually reached a peak by about 10min. 2. When cellular [Na+] and extracellular [Na+] were each about 30m-equiv./l., the maximum amount of glycine absorbed increased between 1·2- and 3·0-fold on lowering extracellular [K+] from 128 to 10m-equiv./l. 3. When extracellular [Na+] was 150m-equiv./l., the ratio, R, of the cellular to extracellular glycine concentrations increased progressively, from near 1 to about 9, when cellular [Na+] was lowered from 120 to 40m-equiv./l. 4. When cellular [Na+] was almost constant, either at 45 or 70m-equiv./l., R fell about 14-fold when extracellular [Na+] varied from 150 to 16m-equiv./l. 5. Values of R near 0·2 were found when cellular [Na+] was about four times as large as extracellular [Na+]. 6. R fell about threefold when the cells were put with 12mm- instead of 0·9mm-glycine. 7. The results were taken to imply that, under these conditions, the spontaneous movements of both Na+ and K+ across the cell membrane, down their respective concentration gradients, served to concentrate the glycine in the tumour cells (Christensen's hypothesis).
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- CHRISTENSEN H. N., RIGGS T. R., FISCHER H., PALATINE I. M. Amino acid concentration by a free cell neoplasm; relations among amino acids. J Biol Chem. 1952 Sep;198(1):1–15. [PubMed] [Google Scholar]
- CRANE R. K. Hypothesis for mechanism of intestinal active transport of sugars. Fed Proc. 1962 Nov-Dec;21:891–895. [PubMed] [Google Scholar]
- Eddy A. A. A net gain of sodium ions and a net loss of potassium ions accompanying the uptake of glycine by mouse ascites-tumour cells in the presence of sodium cyanide. Biochem J. 1968 Jun;108(2):195–206. doi: 10.1042/bj1080195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eddy A. A., Mulcahy M. F., Thomson P. J. The effects of sodium ions and potassium ions on glycine uptake by mouse ascites-tumour cells in the presence and absence of selected metabolic inhibitors. Biochem J. 1967 Jun;103(3):863–876. doi: 10.1042/bj1030863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RIGGS T. R., WALKER L. M., CHRISTENSEN H. N. Potassium migration and amino acid transport. J Biol Chem. 1958 Dec;233(6):1479–1484. [PubMed] [Google Scholar]
- VIDAVER G. A. GLYCINE TRANSPORT BY HEMOLYZED AND RESTORED PIGEON RED CELLS. Biochemistry. 1964 Jun;3:795–799. doi: 10.1021/bi00894a011. [DOI] [PubMed] [Google Scholar]
- Wheeler K. P., Inui Y., Hollenberg P. F., Eavenson E., Christensen H. N. Relation of amino acid transport to sodium-ion concentration. Biochim Biophys Acta. 1965 Nov 29;109(2):620–622. doi: 10.1016/0926-6585(65)90191-3. [DOI] [PubMed] [Google Scholar]


