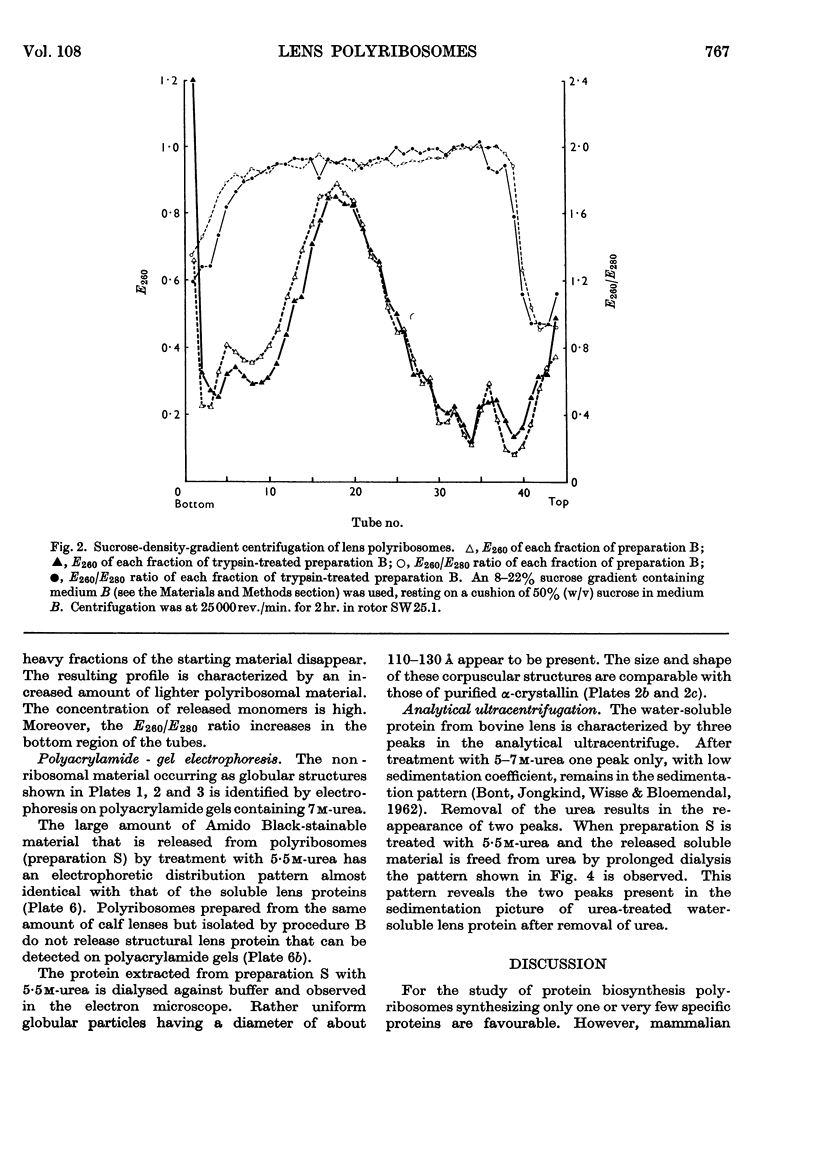
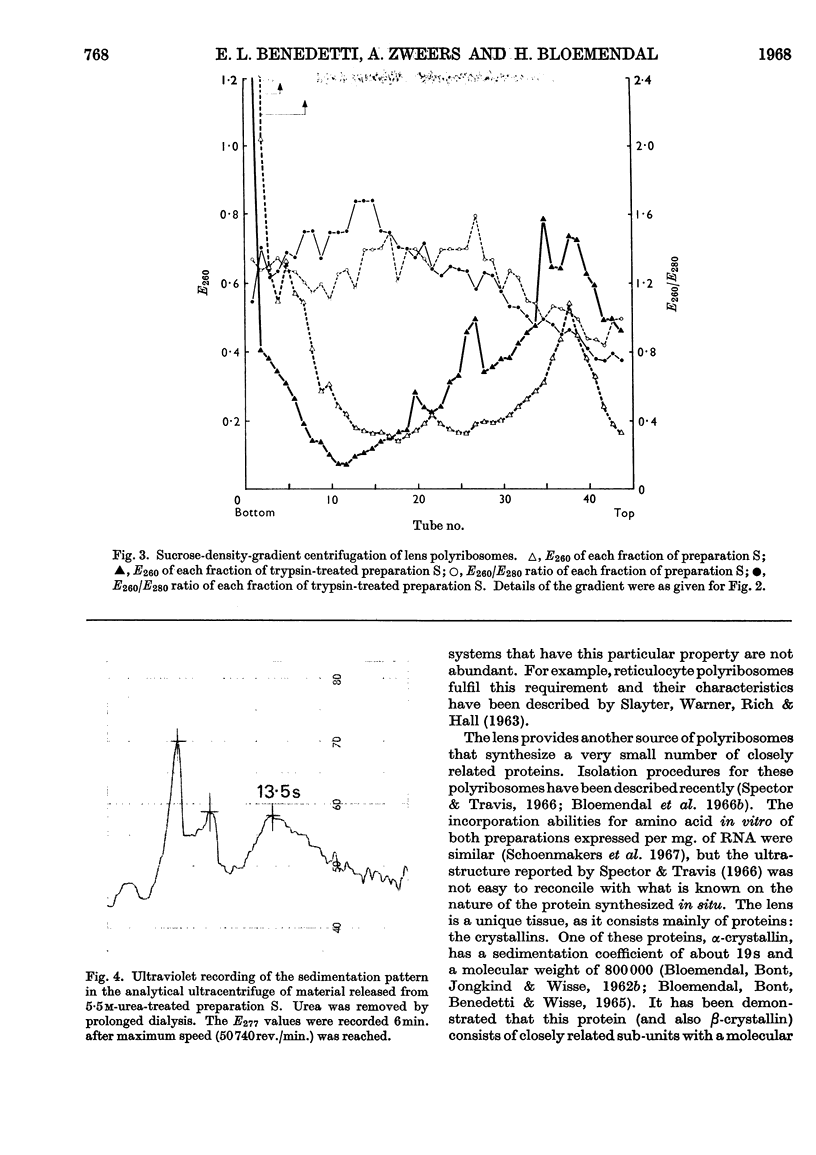
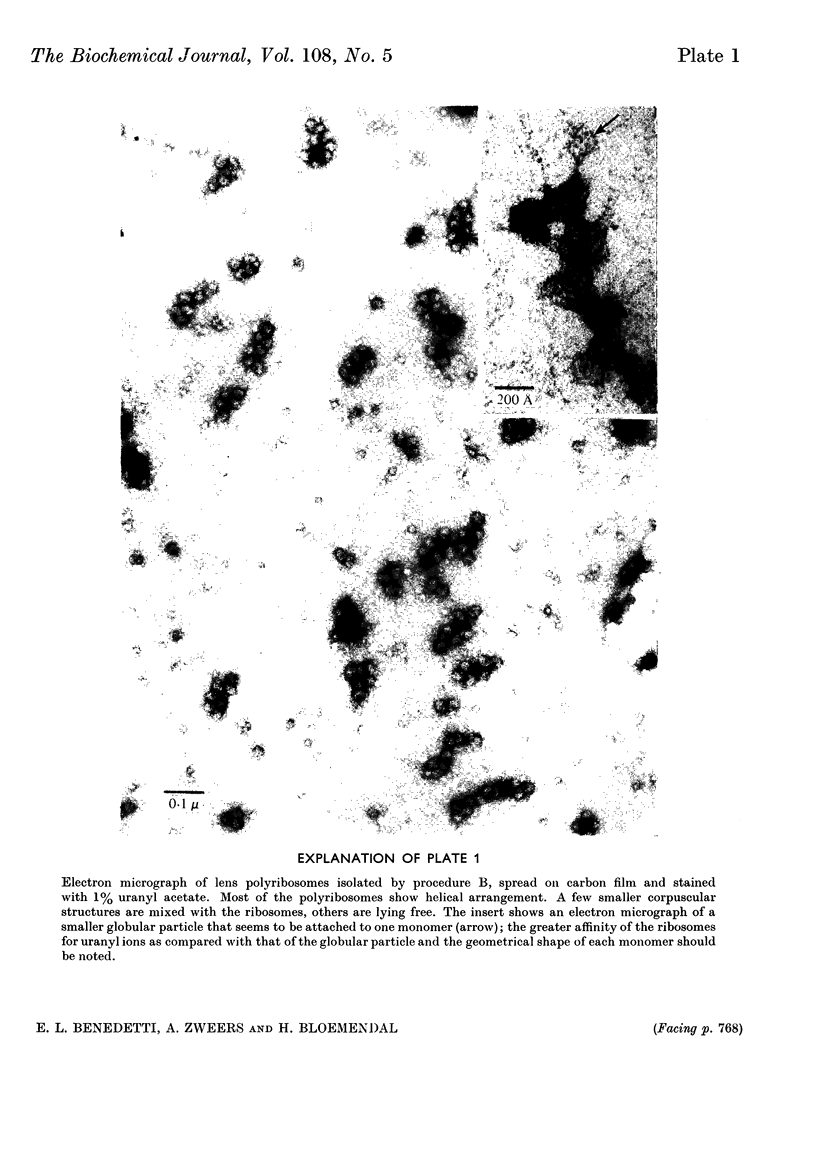
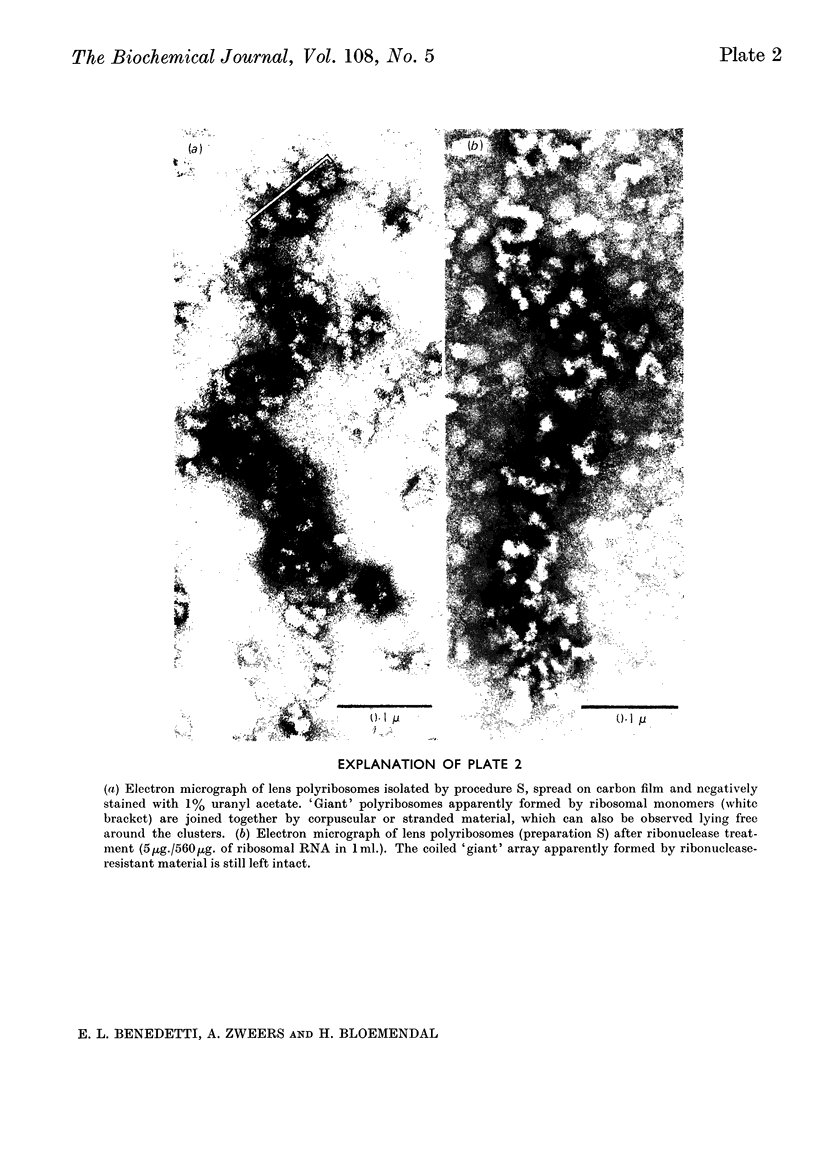
Abstract
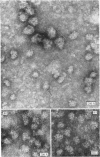
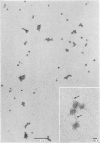
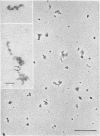
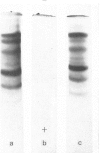
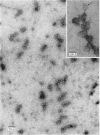
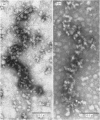
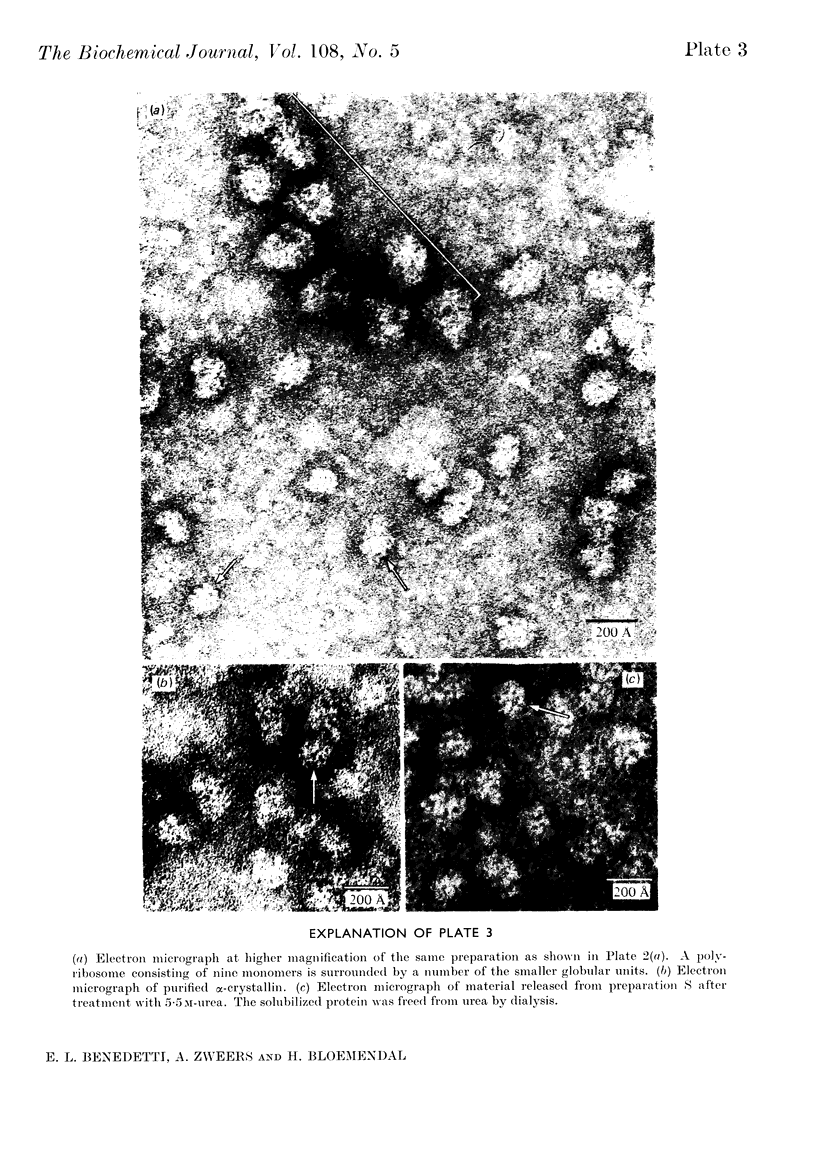
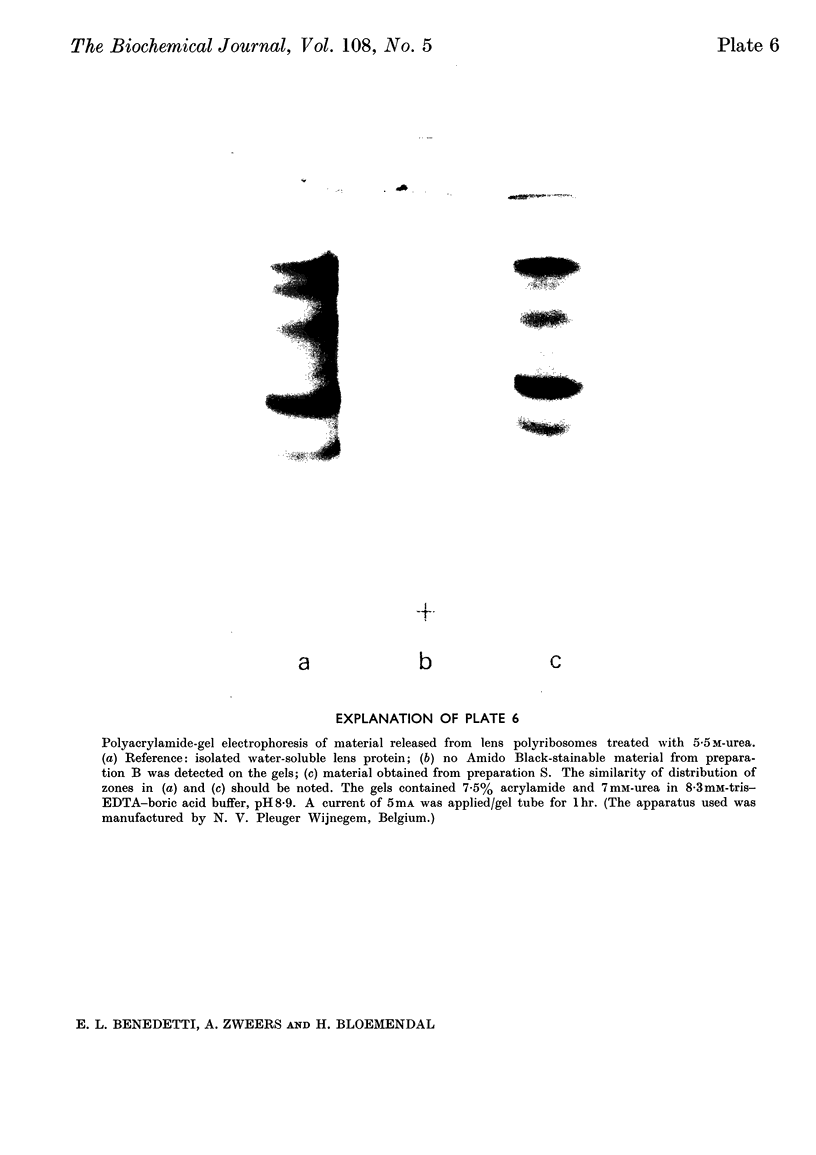
Epithelial cells and the outer cortex from lenses of 1-day-old calves contain polyribosomes occurring in clusters of five to ten monomers. The structure of these clusters is not affected by trypsin treatment up to 10μg./ml. of ribosomal suspension. When the whole lens is used as starting material the polyribosomal preparations are strongly contaminated with non-ribosomal material, which gives rise to ribosomal aggregates having the appearance of giant polyribosomes. These structures are sensitive to trypsin treatment but resist ribonuclease treatment. The electrophoretic pattern of the contaminating material resembles that of water-soluble lens protein.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ARONSSON T., GRONWALL A. Improved separation of serum proteins in paper electrophoresis: a new electrophoresis buffer. Scand J Clin Lab Invest. 1957;9(4):338–341. doi: 10.1080/00365515709079983. [DOI] [PubMed] [Google Scholar]
- BENEDETTI E. L., BLOEMENDAL H., BONT W. S. POLYRIBOSOMES ISOL'ES 'A PARTIR DU FOIE DE RAT. C R Hebd Seances Acad Sci. 1964 Aug 10;259:1353–1356. [PubMed] [Google Scholar]
- BLOEMENDAL H., BONT W. S., JONGKIND J. F., WISSE J. H. 'Purity' of alpha-crystallin. Nature. 1962 Feb 3;193:437–439. doi: 10.1038/193437a0. [DOI] [PubMed] [Google Scholar]
- BLOEMENDAL H., BONT W. S., JONGKIND J. F., WISSE J. H. Splitting and recombination of alpha-crystallin. Exp Eye Res. 1962 Jun;1:300–305. doi: 10.1016/s0014-4835(62)80015-3. [DOI] [PubMed] [Google Scholar]
- BLOEMENDAL H., ten CATE Isolation and properties of alpha-crystallin from the bovine lens. Arch Biochem Biophys. 1959 Oct;84:512–527. doi: 10.1016/0003-9861(59)90612-5. [DOI] [PubMed] [Google Scholar]
- BONT W. S., JONGKIND J. F., WISSE J. H., BLOEMENDAL H. The effect of urea on lens proteins. Biochim Biophys Acta. 1962 May 21;59:512–514. doi: 10.1016/0006-3002(62)90216-0. [DOI] [PubMed] [Google Scholar]
- BREUER C. B., DAVIES M. C., FLORINI J. R. AMINO ACID INCORPORATION INTO PROTEIN BY CELL-FREE PREPARATIONS FROM RAT SKELETAL MUSCLE. II. PREPARATION AND PROPERTIES OF MUSCLE RIBOSOMES AND POLYRIBOSOMES. Biochemistry. 1964 Nov;3:1713–1719. doi: 10.1021/bi00899a020. [DOI] [PubMed] [Google Scholar]
- Benedetti E. L., Bont W. S., Bloemendal H. Electron microscopic observation on polyribosomes and endoplasmic reticulum fragments isolated from rat liver. Lab Invest. 1966 Jan;15(1 Pt 1):196–208. [PubMed] [Google Scholar]
- Bloemendal H., Bont W. S., Benedetti E. L., Wisse J. H. On the subunits of alpha-crystallin. Exp Eye Res. 1965 Dec;4(4):319–326. doi: 10.1016/s0014-4835(65)80047-1. [DOI] [PubMed] [Google Scholar]
- Bloemendal H., Bont W. S., Feltkamp C. A. Studies on hypophyseal isografts in mice. II. Properties of polyribosomes. Cancer Res. 1966 Jul;26(7):1497–1501. [PubMed] [Google Scholar]
- Bloemendal H., Schoenmakers J., Zweers A., Matze R., Benedetti E. L. Polyribosomes from calf-lens epithelium. Biochim Biophys Acta. 1966 Jul 20;123(1):217–220. doi: 10.1016/0005-2787(66)90179-1. [DOI] [PubMed] [Google Scholar]
- Bloemendal H., Zweers A., Schoenmakers J. G. Synthesis of lens protein in vitro. 2. Incorporation of phenylalanine directed by poly U. Eur J Biochem. 1968 Mar;4(1):108–111. doi: 10.1111/j.1432-1033.1968.tb00179.x. [DOI] [PubMed] [Google Scholar]
- Britten R. J., Roberts R. B. High-Resolution Density Gradient Sedimentation Analysis. Science. 1960 Jan 1;131(3392):32–33. doi: 10.1126/science.131.3392.32. [DOI] [PubMed] [Google Scholar]
- Fernández-Madrid F. Biochemical and physicochemical characterization of collagen-synthesizing polyribosomes. J Cell Biol. 1967 Apr;33(1):27–42. doi: 10.1083/jcb.33.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heywood S. M., Dowben R. M., Rich A. The identification of polyribosomes synthesizing myosin. Proc Natl Acad Sci U S A. 1967 Apr;57(4):1002–1009. doi: 10.1073/pnas.57.4.1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SLAYTER H. S., WARNER J. R., RICH A., HALL C. E. THE VISUALIZATION OF POLYRIBOSOMAL STRUCTURE. J Mol Biol. 1963 Dec;7:652–657. doi: 10.1016/s0022-2836(63)80112-6. [DOI] [PubMed] [Google Scholar]
- Schoenmakers J. G., Zweers A., Bloemendal H. Synthesis of lens protein in vitro. I. Properties of polyribosomes. Biochim Biophys Acta. 1967 Aug 22;145(1):120–126. doi: 10.1016/0005-2787(67)90660-0. [DOI] [PubMed] [Google Scholar]
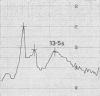
- Van Es W. L., Bont W. S. A recording system for the analytical ultracentrifuge equipped with absorption optics. Anal Biochem. 1966 Nov;17(2):327–343. doi: 10.1016/0003-2697(66)90212-0. [DOI] [PubMed] [Google Scholar]
- Wisse J. H., Zweers A., Jongkind J. F., Bont W. S., Bloemendal H. Further studies on the sub-units of alpha-crystallin. Biochem J. 1966 Apr;99(1):179–188. doi: 10.1042/bj0990179. [DOI] [PMC free article] [PubMed] [Google Scholar]