Abstract
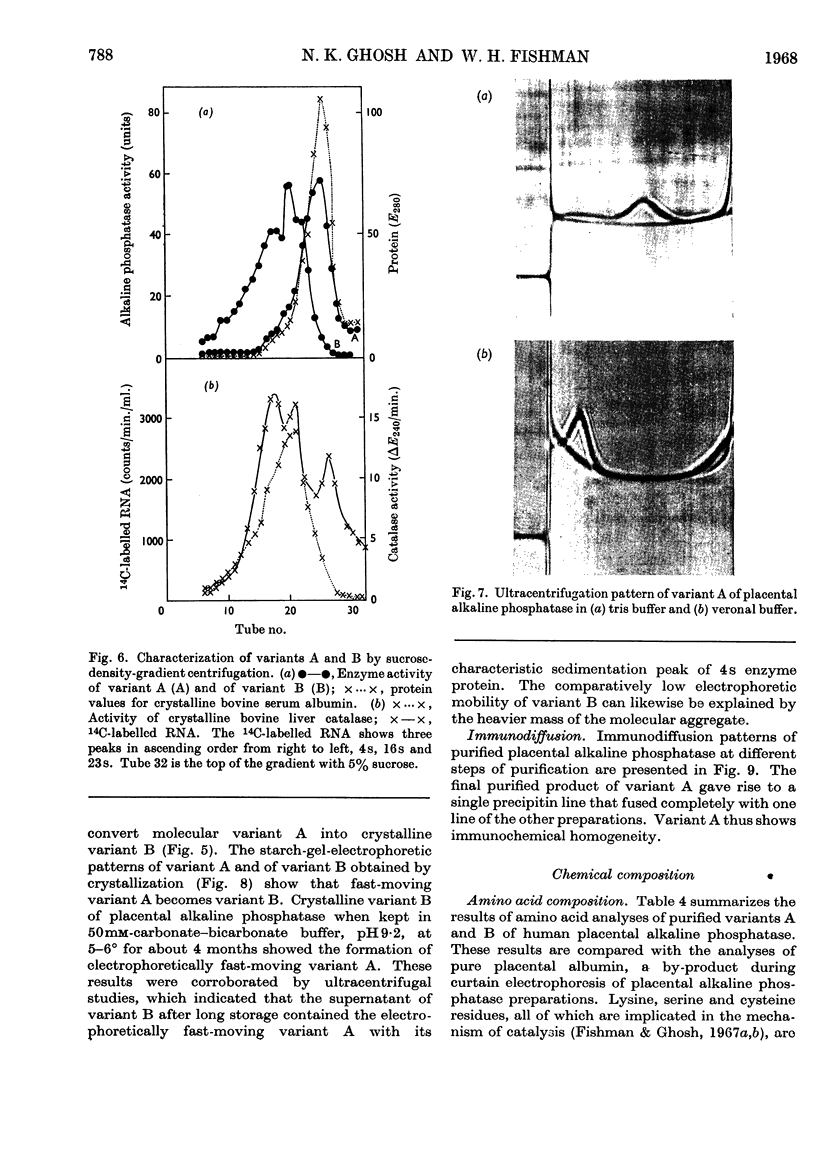
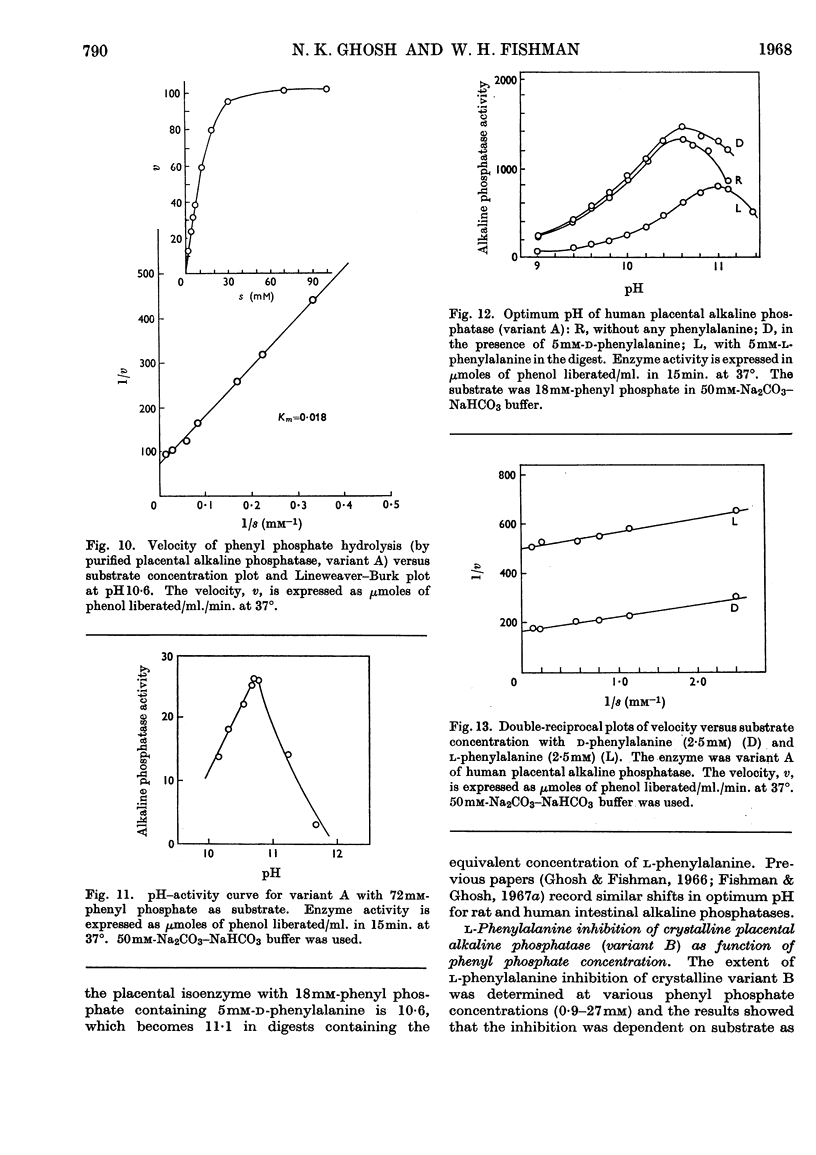
1. Alkaline phosphatase of human placenta was purified by a procedure involving homogenization with tris buffer, pH8·6, extraction with butanol, ammonium sulphate fractionation, exposure to heat, ethanol fractionation, gel filtration, triethylaminoethylcellulose anion-exchange chromatography, continuous curtain electrophoresis on paper and equilibrium dialysis. Methods for both laboratory-scale and large-scale preparation were devised. 2. Two major molecular-weight variants designated A and B were separated by molecular sieving with Sephadex G-200 and variant A was purified 4000-fold. 3. Variant B, which comes off the Sephadex G-200 column before variant A, is the electrophoretically slower-moving species on starch gel and is quite heterogeneous. 4. Purified variant A was fairly homogeneous on the basis of electrophoretic studies on starch gel and Sephadex gel, ultracentrifugation and immunodiffusion. 5. The respective molecular weights for variants A and B were 70000 and over 200000 on the basis of sucrose-density-gradient ultracentrifugation. Variant A exhibited a sedimentation coefficient of 4·2s. 6. Crystalline variant B could be converted into fast-moving variant A and vice versa. 7. Kinetic studies indicated no difference between the two variants. These include linear rates of hydrolysis, pH optimum, Michaelis constants and uncompetitive stereospecific l-phenylalanine inhibition. 8. The amino acid compositions of variants A and B and of placental albumin were determined.
Full text
PDF


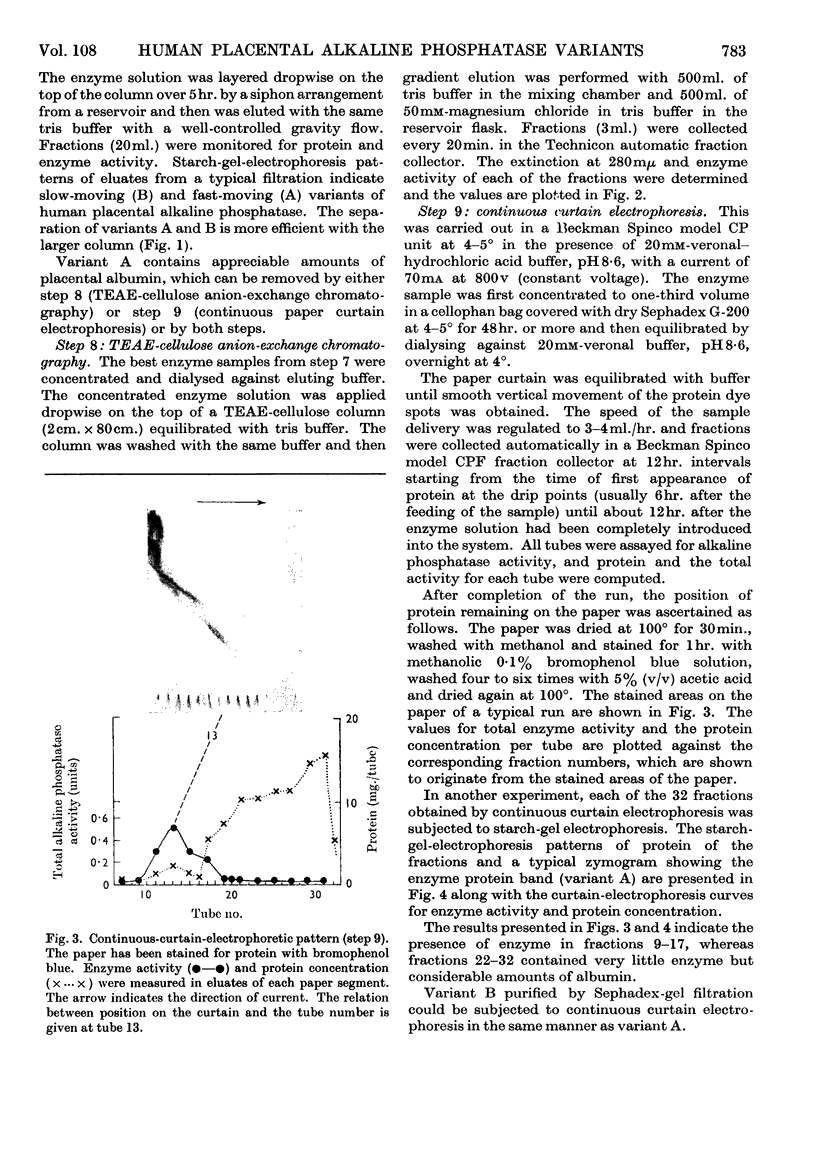
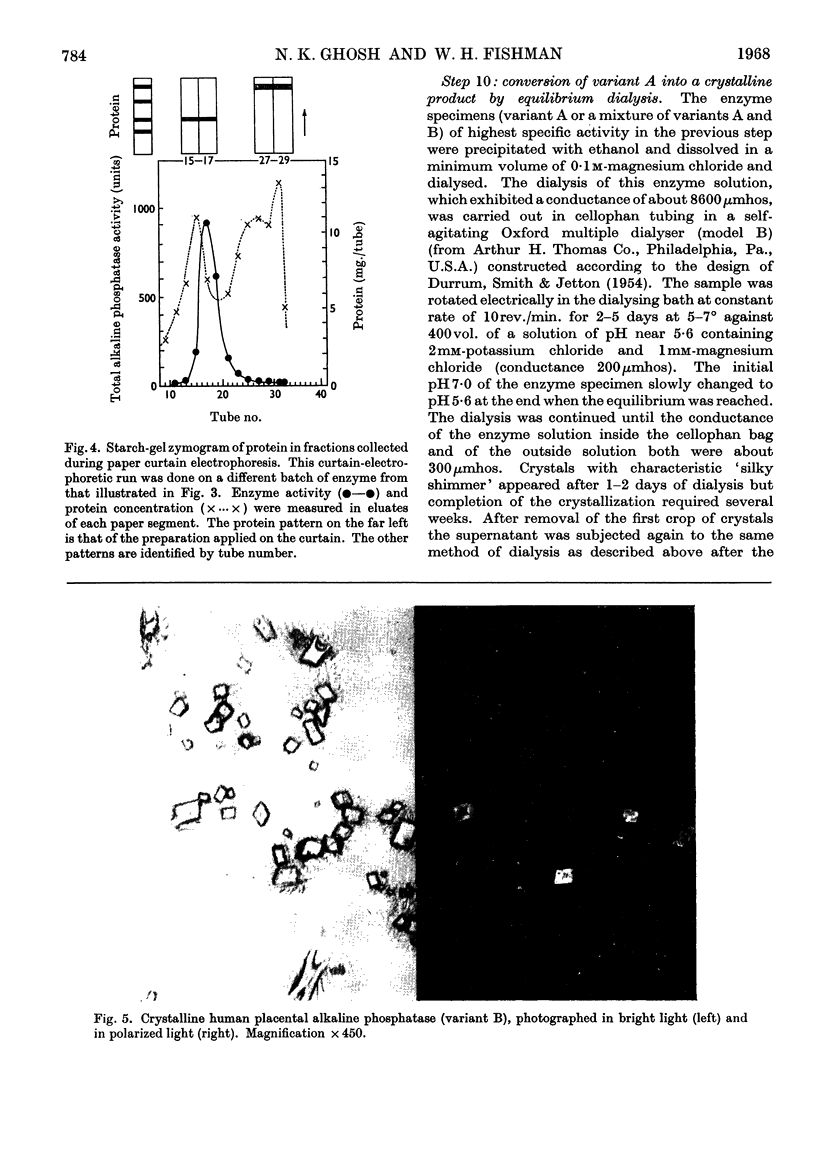

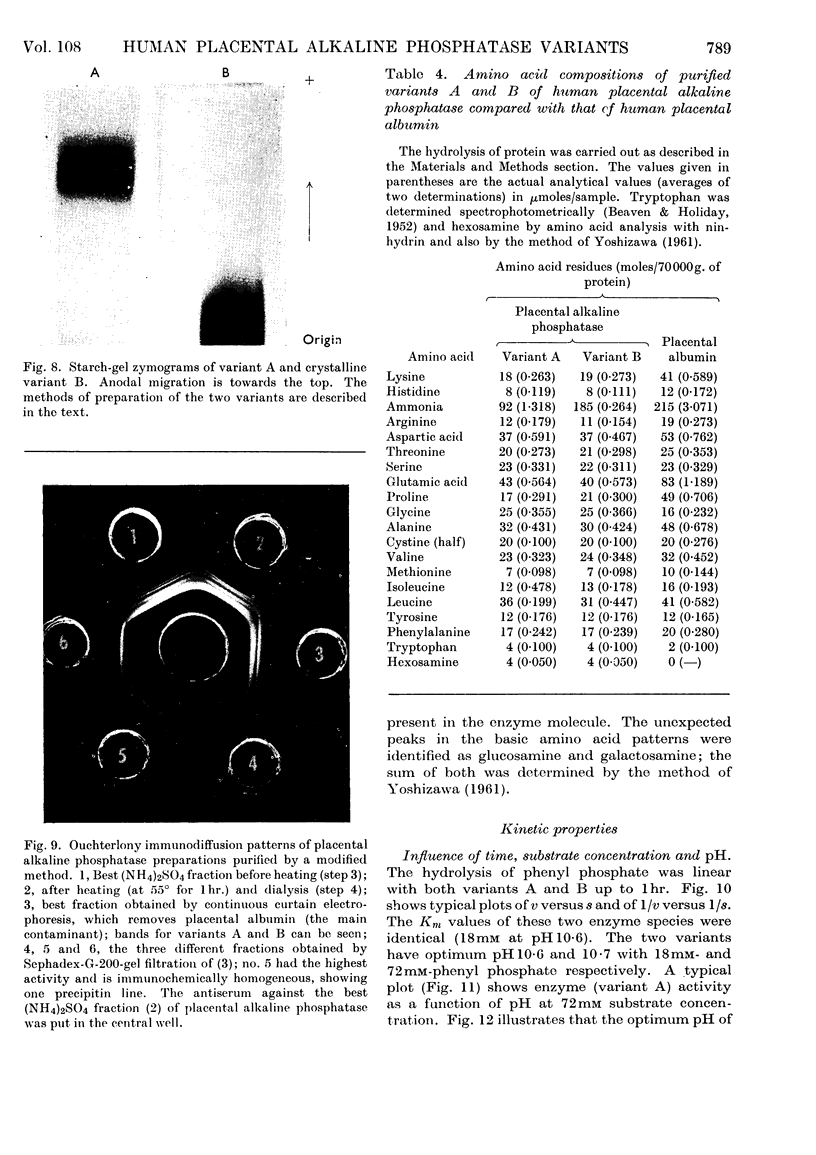


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AHMED Z., KING E. J. Purification of placental alkaline phosphatase. Biochim Biophys Acta. 1960 May 20;40:320–328. doi: 10.1016/0006-3002(60)91357-3. [DOI] [PubMed] [Google Scholar]
- ANDERSON A. D., PATTON R. L. Determination of xanthine oxidase in insects with tetrazolium salts. Science. 1954 Dec 3;120(3127):956–956. doi: 10.1126/science.120.3127.956. [DOI] [PubMed] [Google Scholar]
- FISHMAN W. H., GREEN S., INGLIS N. I. L-phenylalanine: an organ specific, stereospecific inhibitor of human intestinal alkaline phosphatase. Nature. 1963 May 18;198:685–686. doi: 10.1038/198685b0. [DOI] [PubMed] [Google Scholar]
- FISHMAN W. H., GREEN S., INGLIS N. I. Organ-specific behavior exhibited by rat intestine and liver alkaline phosphatase. Biochim Biophys Acta. 1962 Aug 13;62:363–375. doi: 10.1016/0006-3002(62)90266-4. [DOI] [PubMed] [Google Scholar]
- Fishman W. H., Ghosh N. K. Influence of reagents reacting with metal, thiol and amino sites of catalytic activity and l-phenylalanine inhibition of rat intestinal alkaline phosphatase. Biochem J. 1967 Dec;105(3):1163–1170. doi: 10.1042/bj1051163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh N. K., Fishman W. H. On the mechanism of inhibition of intestinal alkaline phosphatase by L-phenylalanine. I. Kinetic studies. J Biol Chem. 1966 Jun 10;241(11):2516–2522. [PubMed] [Google Scholar]
- Ghosh N. K., Goldman S. S., Fishman W. H. Human placental alkaline phosphatase; a sialoprotein. Enzymologia. 1967 Aug 31;33(2):113–124. [PubMed] [Google Scholar]
- Inglis N. R., Ghosh N. K., Fishman W. H. Sephadex G-200 gel electrophoresis of human serum alkaline phosphatases. Anal Biochem. 1968 Mar;22(3):382–386. doi: 10.1016/0003-2697(68)90279-0. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Liss M. Isolation of a heat-stable crystalline protein from psoriatic scales. Biochemistry. 1965 Dec;4(12):2705–2711. doi: 10.1021/bi00888a021. [DOI] [PubMed] [Google Scholar]
- MARTIN R. G., AMES B. N. A method for determining the sedimentation behavior of enzymes: application to protein mixtures. J Biol Chem. 1961 May;236:1372–1379. [PubMed] [Google Scholar]
- NYMAN P. O. Purification and properties of carbonic anhydrase from human erythrocytes. Biochim Biophys Acta. 1961 Sep 2;52:1–12. doi: 10.1016/0006-3002(61)90898-8. [DOI] [PubMed] [Google Scholar]
- REIF A. E., NORRIS H. J. A system for quantitative determination of cytotoxic activity of antisera to ascites tumor cells. Cancer Res. 1960 Sep;20:1235–1244. [PubMed] [Google Scholar]
- SMITHIES O. An improved procedure for starch-gel electrophoresis: further variations in the serum proteins of normal individuals. Biochem J. 1959 Mar;71(3):585–587. doi: 10.1042/bj0710585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STOLBACH L. L., NISSELBAUM J. S., FISHMAN W. H. A simplified technic for measuring serum acid phosphatase of prostatic origin (Fishman-Lerner method). Am J Clin Pathol. 1958 Apr;29(4):379–384. doi: 10.1093/ajcp/29.4_ts.379. [DOI] [PubMed] [Google Scholar]
- Störmer F. C. Isolation of crystalline pH 6 acetolactate-forming enzyme from Aerobacter aerogenes. J Biol Chem. 1967 Apr 25;242(8):1756–1759. [PubMed] [Google Scholar]
- YOSIZAWA Z. Fractional estimation of glucosamine and galactosamine by paper chromatography. 3. Tohoku J Exp Med. 1961 Jun 25;74:69–75. doi: 10.1620/tjem.74.69. [DOI] [PubMed] [Google Scholar]
- Yonetani T., Chance B., Kajiwara S. Crystalline cytochrome c peroxidase and complex ES. J Biol Chem. 1966 Jun 25;241(12):2981–2982. [PubMed] [Google Scholar]