Abstract
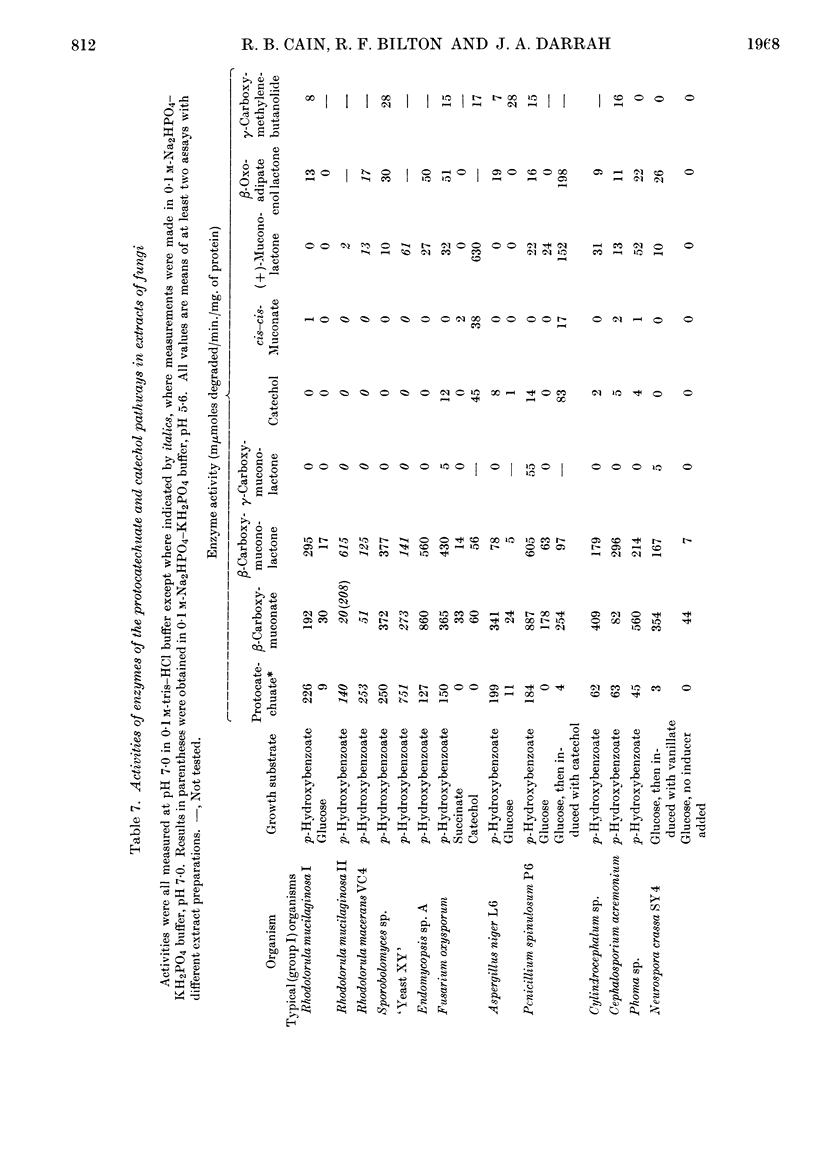
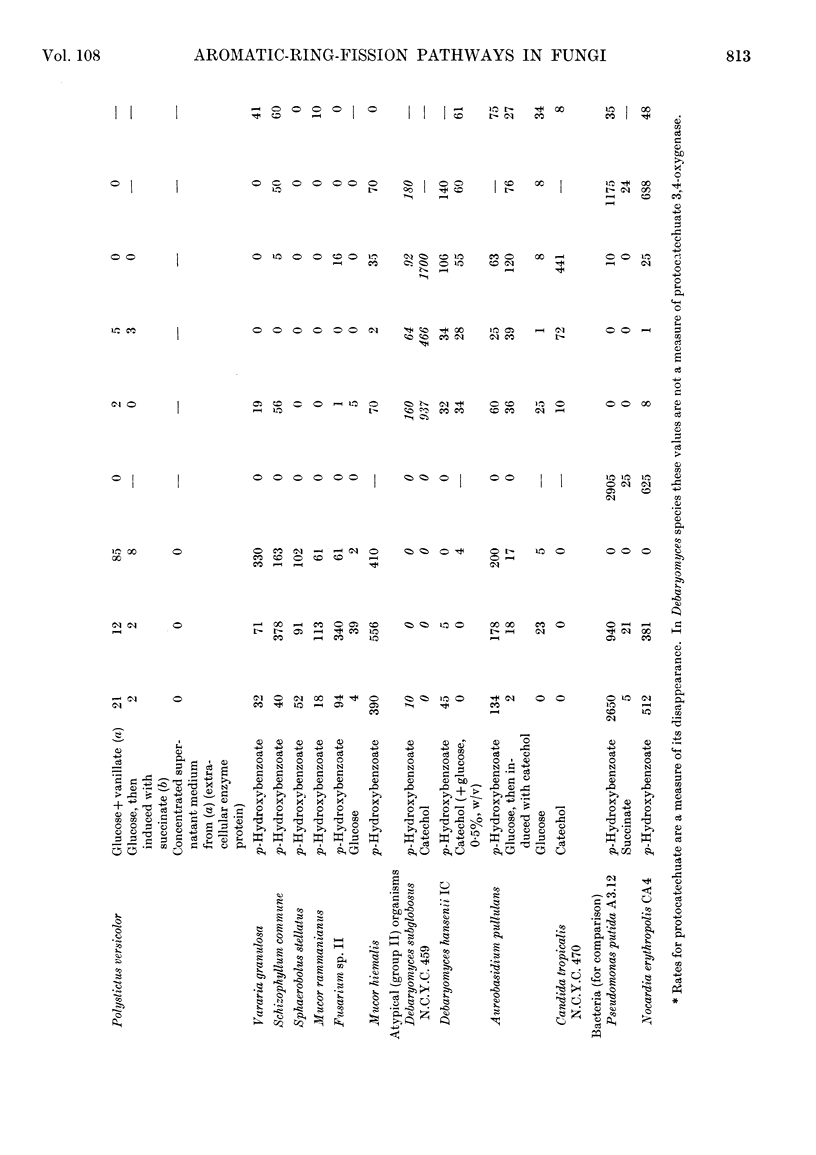
1. The metabolic pathways of aromatic-ring fission were examined in a range of fungal genera that utilize several compounds related to lignin. 2. Most of the genera, after growth on p-hydroxybenzoate, protocatechuate or compounds that are degraded to the latter (e.g. caffeate, ferulate or vanillate), rapidly oxidized these compounds, but not catechol. 3. Such genera possessed a protocatechuate 3,4-oxygenase and accumulated β-carboxymuconate as the product of protocatechuate oxidation. This enzyme had a high pH optimum in most organisms; the Rhodotorula enzyme was competitively inhibited by catechol. 4. β-Carboxymuconate was converted by all competent fungi into β-carboxymuconolactone, which was isolated and characterized. None of the fungi produced or utilized at significant rates the corresponding bacterial intermediate γ-carboxymuconolactone. 5. The lactonizing enzymes of Rhodotorula and Neurospora crassa had a pH optimum near 5·5 and approximate molecular weights of 19000 and 190000 respectively. 6. The fungi did not degrade the isomeric (+)-muconolactone, γ-carboxymethylenebutanolide or β-oxoadipate enol lactone at significant rates, and thus differ radically from bacteria, where β-oxoadipate enol lactone is the precursor of β-oxoadipate in all strains examined. 7. The end product of β-carboxymuconolactone metabolism by extracts was β-oxoadipate. 8. Evidence for a coenzyme A derivative of β-oxoadipate was found during further metabolism of this keto acid. 9. A few anomalous fungi, after growth on p-hydroxybenzoate, had no protocatechuate 3,4-oxygenase, but possessed all the enzymes of the catechol pathway. Catechol was detected in the growth medium in one instance. 10. A strain of Penicillium sp. formed pyruvate but no β-oxoadipate from protocatechuate, suggesting the existence also of a `meta' type of ring cleavage among fungi.
Full text
PDF






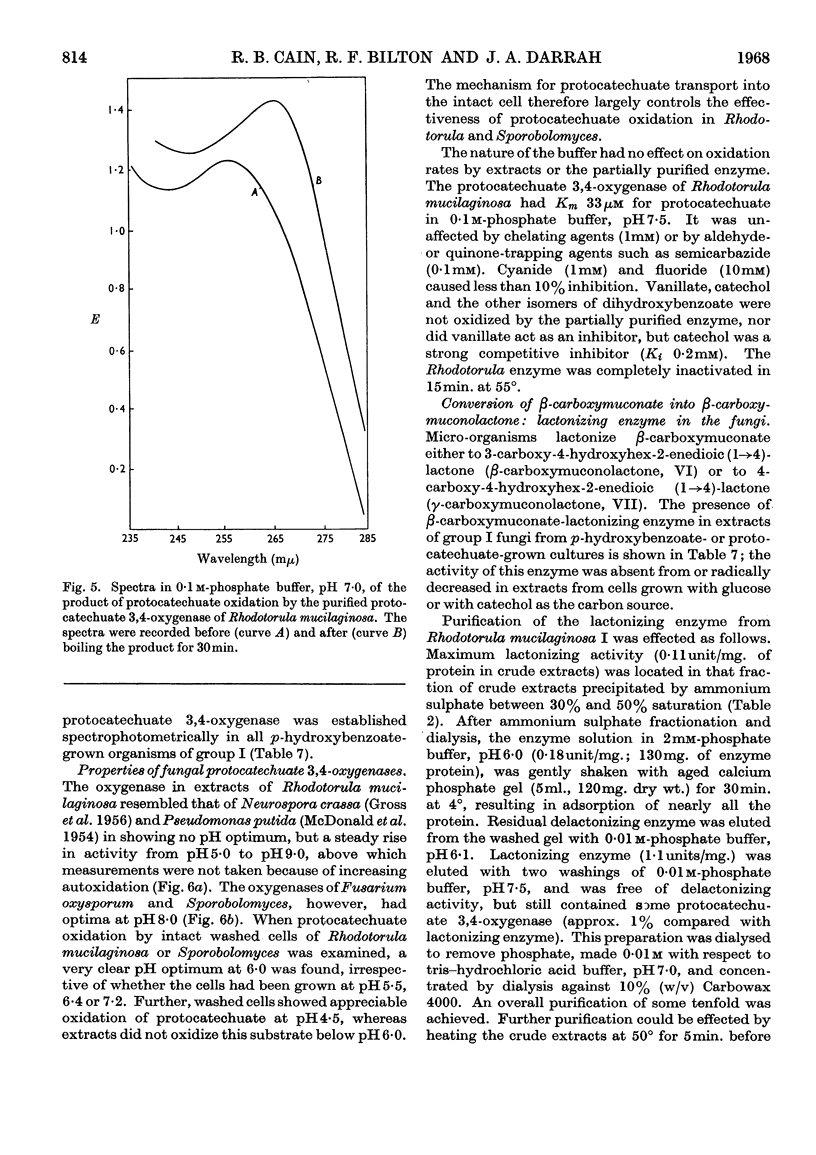
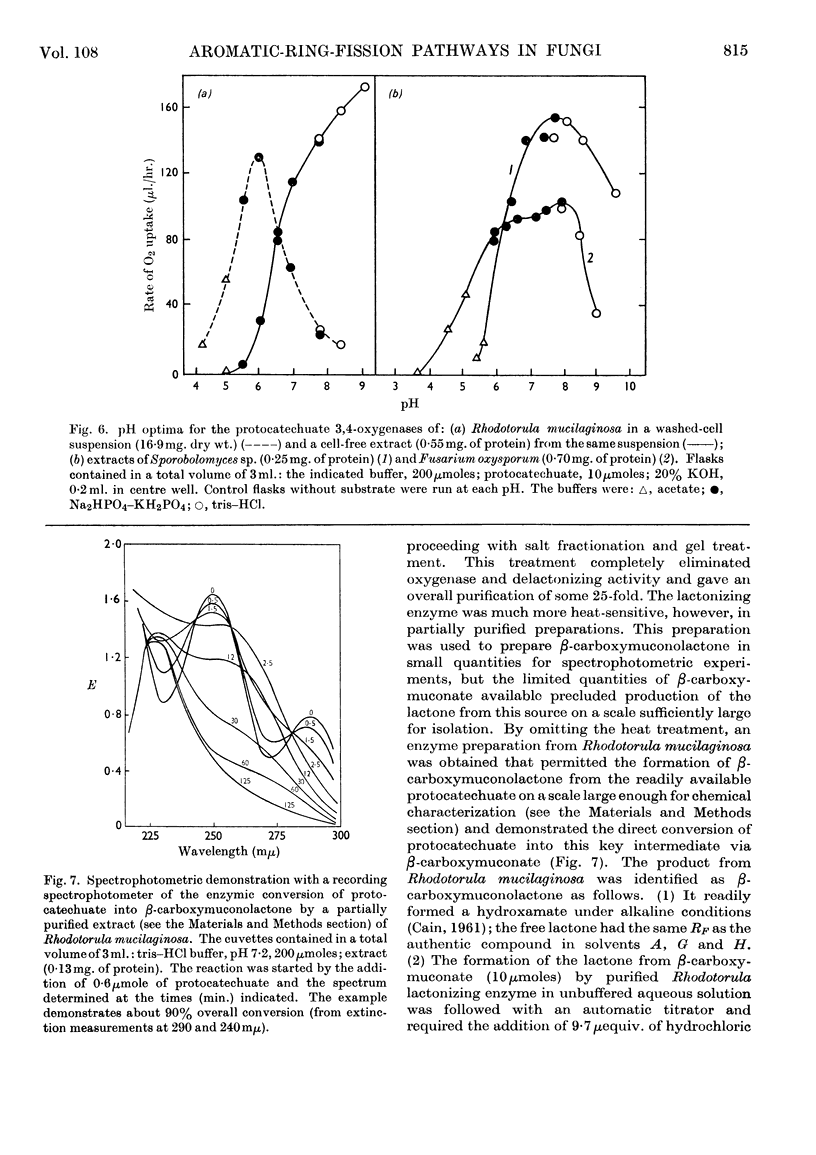

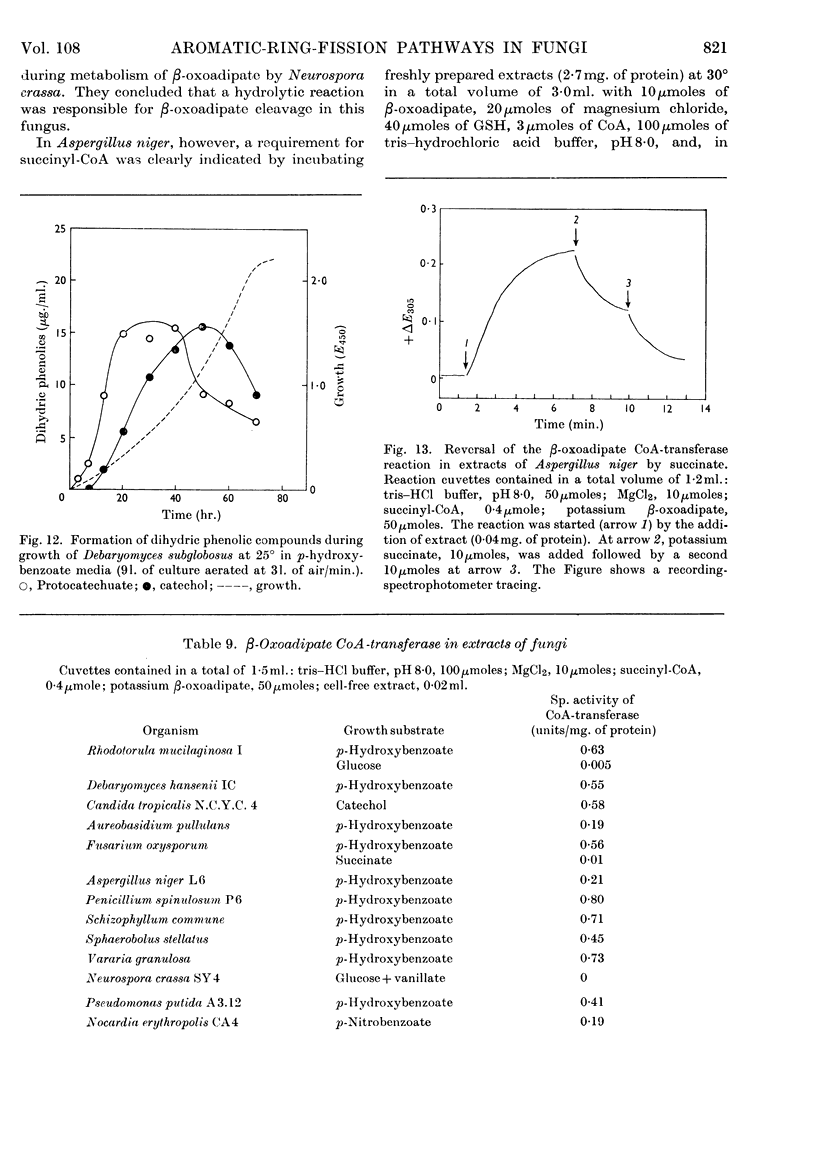


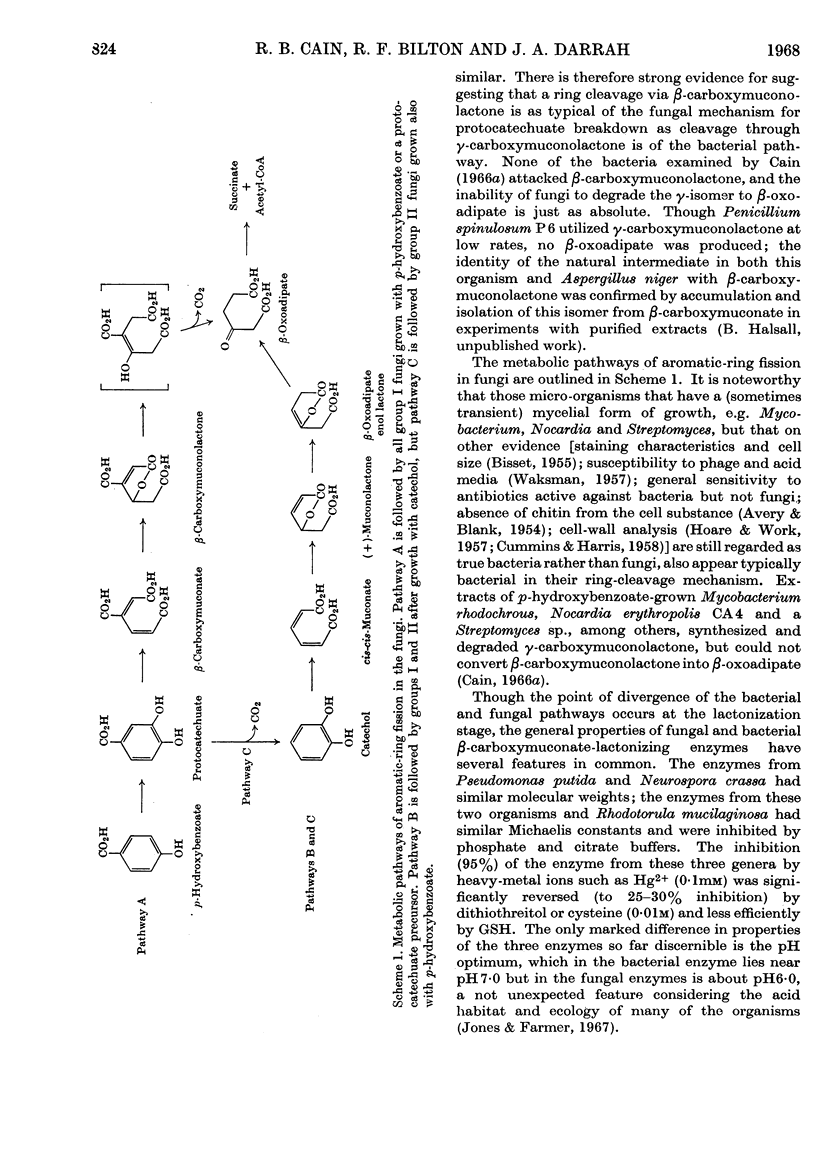




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AVERY R. J., BLANK F. On the chemical composition of the cell walls of the Actinomycetales and its relation to their systematic position. Can J Microbiol. 1954 Oct;1(2):140–143. doi: 10.1139/m55-018. [DOI] [PubMed] [Google Scholar]
- Bayly R. C., Dagley S., Gibson D. T. The metabolism of cresols by species of Pseudomonas. Biochem J. 1966 Nov;101(2):293–301. doi: 10.1042/bj1010293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CAIN R. B., CARTWRIGHT N. J. On the properties of some aromatic ring-opening enzymes of species of the genus Nocardia. Biochim Biophys Acta. 1960 Jan 15;37:197–213. doi: 10.1016/0006-3002(60)90225-0. [DOI] [PubMed] [Google Scholar]
- CAIN R. B. New aromatic ring-splitting enzyme, protocatechuic acid-4:5-oxygenase. Nature. 1962 Mar 3;193:842–844. doi: 10.1038/193842a0. [DOI] [PubMed] [Google Scholar]
- CAIN R. B. The metabolism of protocatechuic acid by a vibrio. Biochem J. 1961 May;79:298–312. doi: 10.1042/bj0790298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CAIN R. B. The metabolism of protocatechuic acid by a vibrio. Biochem J. 1961 May;79:298–312. doi: 10.1042/bj0790298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CARTWRIGHT N. J., CAIN R. B. Bacterial degradation of the nitrobenzoic acids. Biochem J. 1959 Feb;71(2):248–261. doi: 10.1042/bj0710248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COOPER J., SRERE P. A., TABACHNICK M., RACKER E. The oxidative pentose phosphate cycle. II. Quantitative determination of intermediates and enzymes. Arch Biochem Biophys. 1958 Apr;74(2):306–314. doi: 10.1016/0003-9861(58)90002-x. [DOI] [PubMed] [Google Scholar]
- CUMMINS C. S., HARRIS H. Studies on the cell-wall composition and taxonomy of Actinomycetales and related groups. J Gen Microbiol. 1958 Feb;18(1):173–189. doi: 10.1099/00221287-18-1-173. [DOI] [PubMed] [Google Scholar]
- Cain R. B., Farr D. R. Metabolism of arylsulphonates by micro-organisms. Biochem J. 1968 Feb;106(4):859–877. doi: 10.1042/bj1060859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cain R. B., Tranter E. K., Darrah J. A. The utilization of some halogenated aromatic acids by Nocardia. Oxidation and metabolism. Biochem J. 1968 Jan;106(1):211–227. doi: 10.1042/bj1060211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cain R. B. Utilization of anthranilic and nitrobenzoic acids by Nocardia opaca and a flavobacterium. J Gen Microbiol. 1966 Feb;42(2):219–235. doi: 10.1099/00221287-42-2-219. [DOI] [PubMed] [Google Scholar]
- Campbell W. G. The chemistry of the white rots of wood: The effect on wood substance of Armillaria mellea (Vahl.) Fr., Polyporus hispidus (Bull.) Fr., and Stereum hirsutum Fr. Biochem J. 1931;25(6):2023–2027. doi: 10.1042/bj0252023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cartwright N. J., Buswell J. A. The separation of vanillate O-demethylase from protocatechuate 3,4-oxygenase by ultracentrifugation. Biochem J. 1967 Nov;105(2):767–770. doi: 10.1042/bj1050767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cartwright N. J., Smith A. R. Bacterial attack on phenolic ethers: An enzyme system demethylating vanillic acid. Biochem J. 1967 Mar;102(3):826–841. doi: 10.1042/bj1020826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cánovas J. L., Ornston L. N., Stanier R. Y. Evolutionary significance of metabolic control systems. The beta-ketoadipate pathway provides a case history in bacteria. Science. 1967 Jun 30;156(3783):1695–1699. doi: 10.1126/science.156.3783.1695. [DOI] [PubMed] [Google Scholar]
- Cánovas J. L., Stanier R. Y. Regulation of the enzymes of the beta-ketoadipate pathway in Moraxella calcoacetica. 1. General aspects. Eur J Biochem. 1967 May;1(3):289–300. doi: 10.1007/978-3-662-25813-2_40. [DOI] [PubMed] [Google Scholar]
- DAGLEY S., CHAPMAN P. J., GIBSON D. T., WOOD J. M. DEGRADATION OF THE BENZENE NUCLEUS BY BACTERIA. Nature. 1964 May 23;202:775–778. doi: 10.1038/202775a0. [DOI] [PubMed] [Google Scholar]
- EVANS W. C., SMITH B. S. W., LINSTEAD R. P., ELVIDGE J. A. Chemistry of the oxidative metabolism of certain aromatic compounds by micro-organisms. Nature. 1951 Nov 3;168(4279):772–775. doi: 10.1038/168772a0. [DOI] [PubMed] [Google Scholar]
- Evans W. C. Oxidation of phenol and benzoic acid by some soil bacteria. Biochem J. 1947;41(3):373–382. doi: 10.1042/bj0410373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GROSS S. R., GAFFORD R. D., TATUM E. L. The metabolism of protocatechuic acid by Neurospora. J Biol Chem. 1956 Apr;219(2):781–796. [PubMed] [Google Scholar]
- HENDERSON M. E. A study of the metabolism of phenolic compounds by soil fungi using spore suspensions. J Gen Microbiol. 1956 Jul;14(3):684–691. doi: 10.1099/00221287-14-3-684. [DOI] [PubMed] [Google Scholar]
- HENDERSON M. E., FARMER V. C. Utilization by soil fungi of p-hydroxybenzaidehyde, ferulic acid, syringaldehyde and vanillin. J Gen Microbiol. 1955 Feb;12(1):37–46. doi: 10.1099/00221287-12-1-37. [DOI] [PubMed] [Google Scholar]
- HENDERSON M. E. Metabolism of methoxylated aromatic compounds by soil fungi. J Gen Microbiol. 1957 Jun;16(3):686–695. doi: 10.1099/00221287-16-3-686. [DOI] [PubMed] [Google Scholar]
- HENDERSON M. E. The influence of trace elements on the metabolism of aromatic compounds by soil fungi. J Gen Microbiol. 1960 Oct;23:307–313. doi: 10.1099/00221287-23-2-307. [DOI] [PubMed] [Google Scholar]
- HENDERSON M. E. The metabolism of aromatic compounds related to lignin by some hyphomycetes and yeast-like fungi of soil. J Gen Microbiol. 1961 Sep;26:155–165. doi: 10.1099/00221287-26-1-155. [DOI] [PubMed] [Google Scholar]
- HOARE D. S., WORK E. The stereoisomers of alpha epsilon-diaminopimelic acid. II. Their distribution in the bacterial order Actinomycetales and in certain Eubacteriales. Biochem J. 1957 Mar;65(3):441–447. doi: 10.1042/bj0650441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUGHES D. E. A press for disrupting bacteria and other micro-organisms. Br J Exp Pathol. 1951 Apr;32(2):97–109. [PMC free article] [PubMed] [Google Scholar]
- Hegeman G. D. Synthesis of the enzymes of the mandelate pathway by Pseudomonas putida. I. Synthesis of enzymes by the wild type. J Bacteriol. 1966 Mar;91(3):1140–1154. doi: 10.1128/jb.91.3.1140-1154.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosokawa K., Stanier R. Y. Crystallization and properties of p-hydroxybenzoate hydroxylase from Pseudomonas putida. J Biol Chem. 1966 May 25;241(10):2453–2460. [PubMed] [Google Scholar]
- ISHIKAWA H., SCHUBERT W. J., NORD F. F. Investigations on lignins and lignification. 27. The enzymic degradation of softwood lignin by white-rot fungi. Arch Biochem Biophys. 1963 Jan;100:131–139. doi: 10.1016/0003-9861(63)90043-2. [DOI] [PubMed] [Google Scholar]
- KATAGIRI M., HAYAISHI O. Enzymatic degradation of beta-ketoadipic acid. J Biol Chem. 1957 May;226(1):439–448. [PubMed] [Google Scholar]
- KOJIMA Y., ITADA N., HAYAISHI O. Metapyrocatachase: a new catechol-cleaving enzyme. J Biol Chem. 1961 Aug;236:2223–2228. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MACDONALD D. L., STANIER R. Y., INGRAHAM J. L. The enzymatic formation of beta-carboxymuconic acid. J Biol Chem. 1954 Oct;210(2):809–820. [PubMed] [Google Scholar]
- MANDELSTAM J., JACOBY G. A. INDUCTION AND MULTI-SENSITIVE END-PRODUCT REPRESSION IN THE ENZYMIC PATHWAY DEGRADING MANDELATE IN PSEUDOMONAS FLUORESCENS. Biochem J. 1965 Mar;94:569–577. doi: 10.1042/bj0940569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore K., Towers G. H. Degradation of aromatic amino acids by fungi. I. Fate of L-phenylalanine in Schizophyllum commune. Can J Biochem. 1967 Nov;45(11):1659–1665. doi: 10.1139/o67-196. [DOI] [PubMed] [Google Scholar]
- ORNSTON L. N., STANIER R. Y. MECHANISM OF BETA-KETOADIPATE FORMATION BY BACTERIA. Nature. 1964 Dec 26;204:1279–1283. doi: 10.1038/2041279a0. [DOI] [PubMed] [Google Scholar]
- OTTEY L., TATUM E. L. The cleavage of beta-ketoadipic acie by Neurospora crassa. J Biol Chem. 1957 Nov;229(1):77–83. [PubMed] [Google Scholar]
- Ornston L. N., Stanier R. Y. The conversion of catechol and protocatechuate to beta-ketoadipate by Pseudomonas putida. J Biol Chem. 1966 Aug 25;241(16):3776–3786. [PubMed] [Google Scholar]
- Ornston L. N. The conversion of catechol and protocatechuate to beta-ketoadipate by Pseudomonas putida. 3. Enzymes of the catechol pathway. J Biol Chem. 1966 Aug 25;241(16):3795–3799. [PubMed] [Google Scholar]
- Ornston L. N. The conversion of catechol and protocatechuate to beta-ketoadipate by Pseudomonas putida. II. Enzymes of the protocatechuate pathway. J Biol Chem. 1966 Aug 25;241(16):3787–3794. [PubMed] [Google Scholar]
- Ornston L. N. The conversion of catechol and protocatechuate to beta-ketoadipate by Pseudomonas putida. IV. Regulation. J Biol Chem. 1966 Aug 25;241(16):3800–3810. [PubMed] [Google Scholar]
- RIBBONS D. W., EVANS W. C. Oxidative metabolism of protocatechuic acid by certain soil pseudomonads: a new ring-fission mechanism. Biochem J. 1962 Jun;83:482–492. doi: 10.1042/bj0830482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribbons D. W. Metabolism of omicron-cresol by Pseudomonas aeruginosa strain T1. J Gen Microbiol. 1966 Aug;44(2):221–231. doi: 10.1099/00221287-44-2-221. [DOI] [PubMed] [Google Scholar]
- Rothera A. C. Note on the sodium nitro-prusside reaction for acetone. J Physiol. 1908 Dec 15;37(5-6):491–494. doi: 10.1113/jphysiol.1908.sp001285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SISTROM W. R., STANIER R. Y. The mechanism of catechol oxidation by Mycobacterium butyricum. J Bacteriol. 1953 Oct;66(4):404–406. doi: 10.1128/jb.66.4.404-406.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SISTROM W. R., STANIER R. Y. The mechanism of formation of beta-ketoadipic acid by bacteria. J Biol Chem. 1954 Oct;210(2):821–836. [PubMed] [Google Scholar]
- SORENSEN H. Decomposition of lignin by soil bacteria and complex formation between autoxidized lignin and organic nitrogen compounds. J Gen Microbiol. 1962 Jan;27:21–34. doi: 10.1099/00221287-27-1-21. [DOI] [PubMed] [Google Scholar]
- Stanier R. Y., Palleroni N. J., Doudoroff M. The aerobic pseudomonads: a taxonomic study. J Gen Microbiol. 1966 May;43(2):159–271. doi: 10.1099/00221287-43-2-159. [DOI] [PubMed] [Google Scholar]
- Wheelis M. L., Palleroni N. J., Stanier R. Y. The metabolism of aromatic acids by Pseudomonas testosteroni and P. acidovorans. Arch Mikrobiol. 1967;59(1):302–314. doi: 10.1007/BF00406344. [DOI] [PubMed] [Google Scholar]


