Abstract
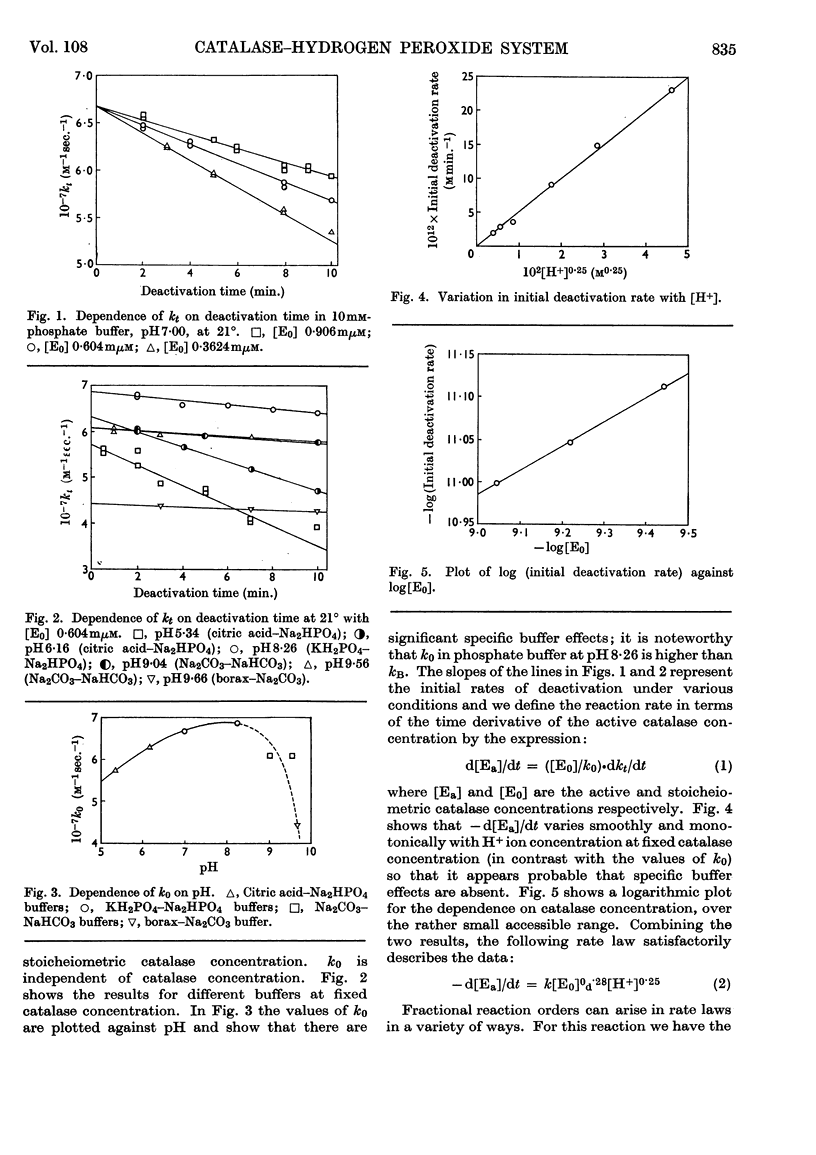
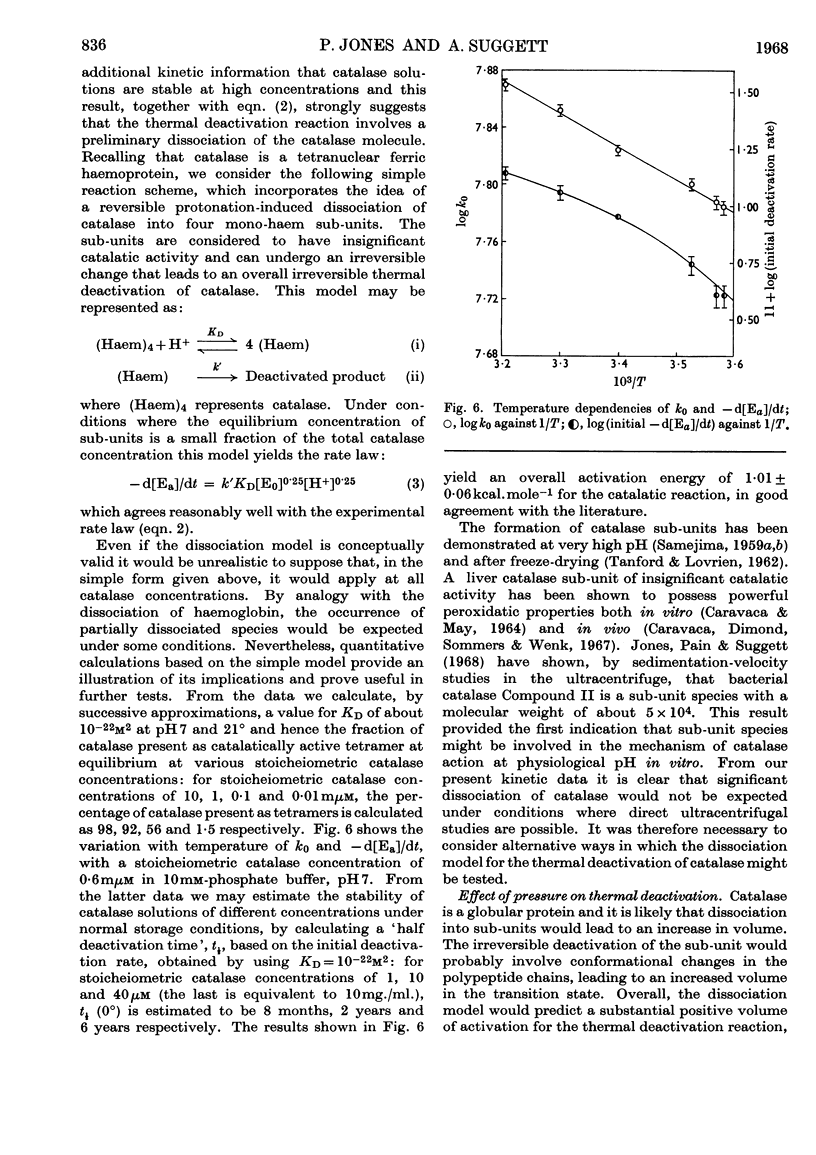
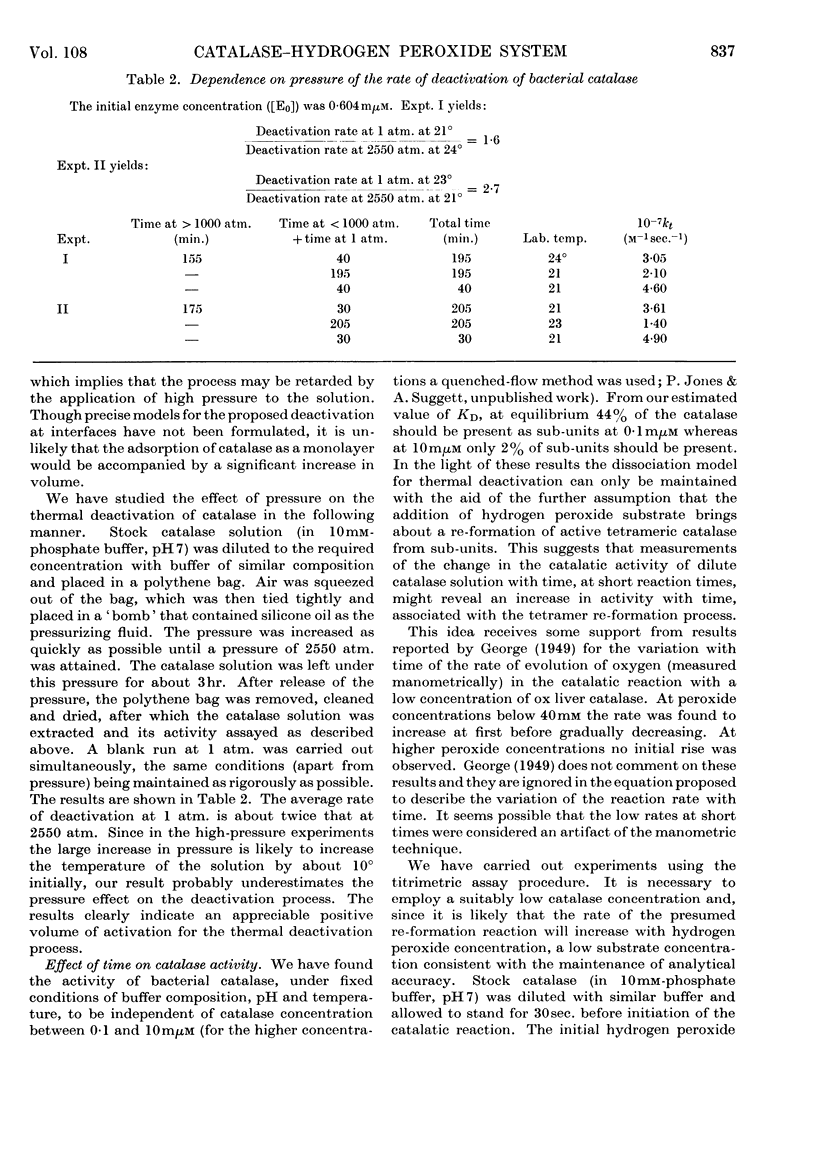
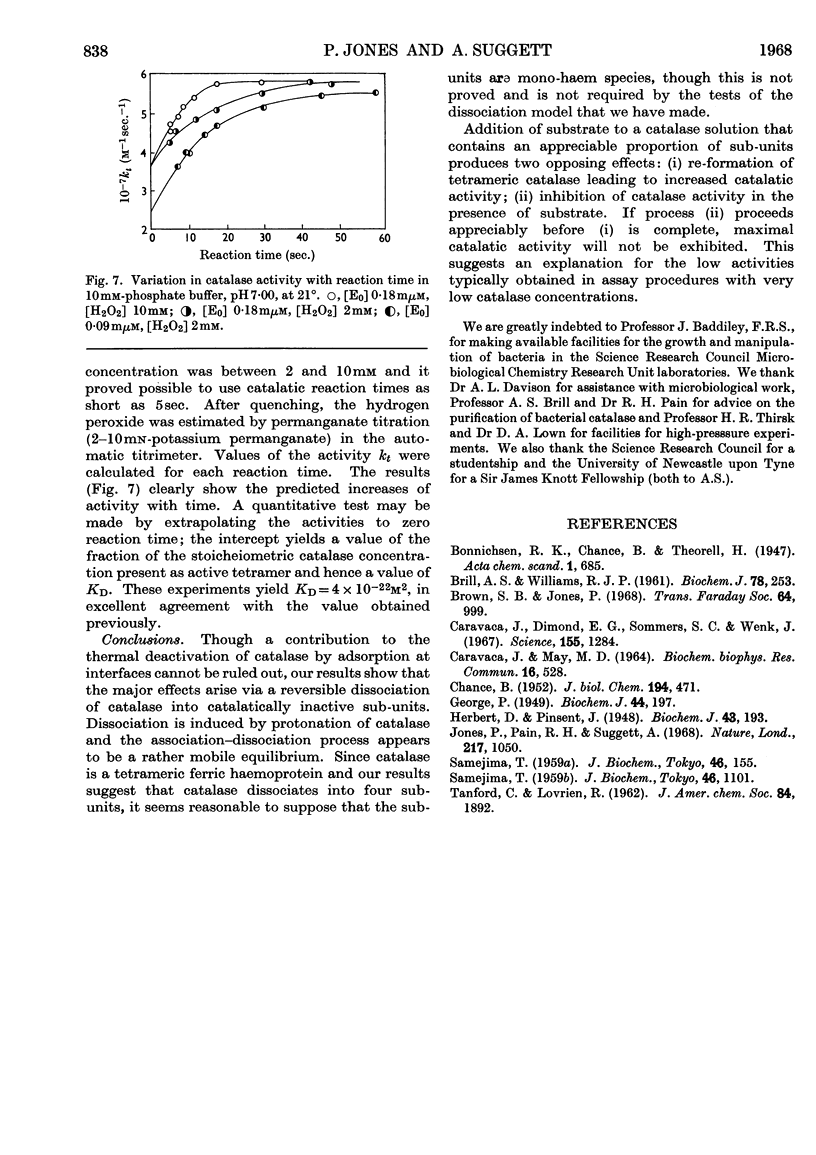
1. Kinetic studies of the thermal deactivation of bacterial catalase in the absence of substrate suggest that the reaction involves a protonation-induced reversible dissociation of catalase into catalatically inactive sub-units, followed by an irreversible transformation of the sub-units into deactivated products. It is possible that the sub-units are mono-haem species. The rate of deactivation decreases with increasing pressure in accordance with the predictions of the proposed model. 2. The results also imply that the addition of hydrogen peroxide substrate induces the re-formation of active catalase. Under appropriate conditions the activity of catalase is found to increase with time in a manner that is quantitatively consistent with the results of deactivation studies.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brill A. S., Williams R. J. Primary compounds of catalase and peroxidase. Biochem J. 1961 Feb;78(2):253–262. doi: 10.1042/bj0780253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHANCE B. Effect of pH upon the reaction kinetics of the enzyme-substrate compounds of catalase. J Biol Chem. 1952 Feb;194(2):471–481. [PubMed] [Google Scholar]
- Caravaca J., Dimond E. G., Sommers S. C., Wenk R. Prevention of induced atherosclerosis by peroxidase. Science. 1967 Mar 10;155(3767):1284–1287. doi: 10.1126/science.155.3767.1284. [DOI] [PubMed] [Google Scholar]
- Caravaca J., May M. D. The isolation and properties of an active peroxidase from hepatocatalase. Biochem Biophys Res Commun. 1964 Aug 11;16(6):528–534. doi: 10.1016/0006-291x(64)90187-1. [DOI] [PubMed] [Google Scholar]
- George P. The effect of the peroxide concentration and other factors on the decomposition of hydrogen peroxide by catalase. Biochem J. 1949;44(2):197–205. doi: 10.1042/bj0440197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbert D., Pinsent J. Crystalline bacterial catalase. Biochem J. 1948;43(2):193–202. doi: 10.1042/bj0430193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones P., Suggett A., Pain R. H. Sub-unit nature of catalase compound II. Nature. 1968 Mar 16;217(5133):1050–1050. doi: 10.1038/2171050a0. [DOI] [PubMed] [Google Scholar]


