Abstract
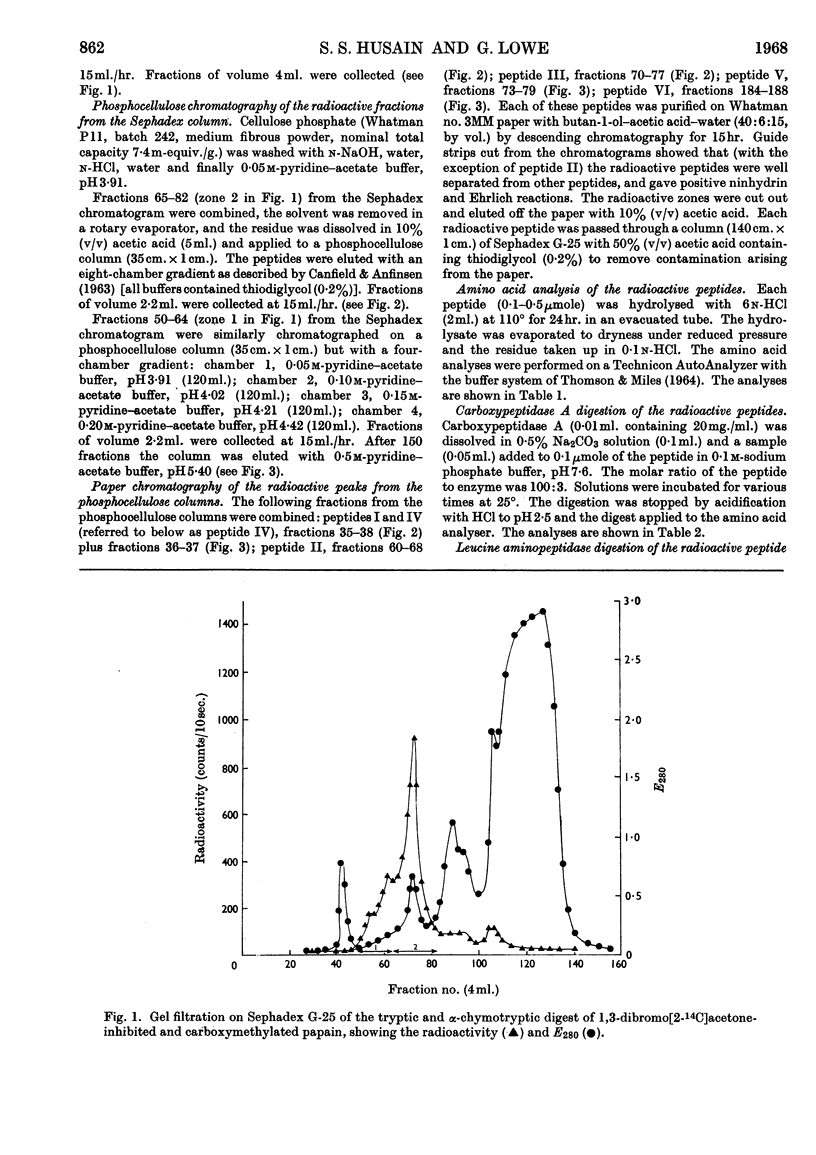
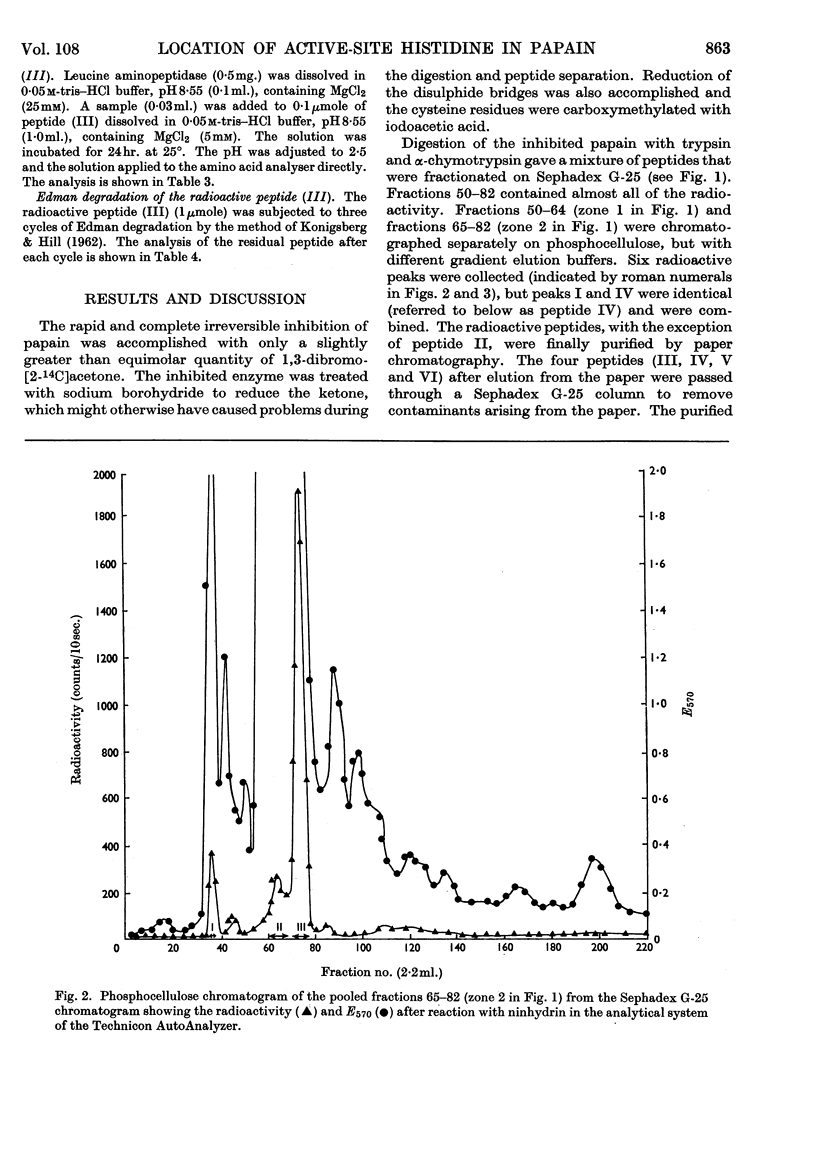
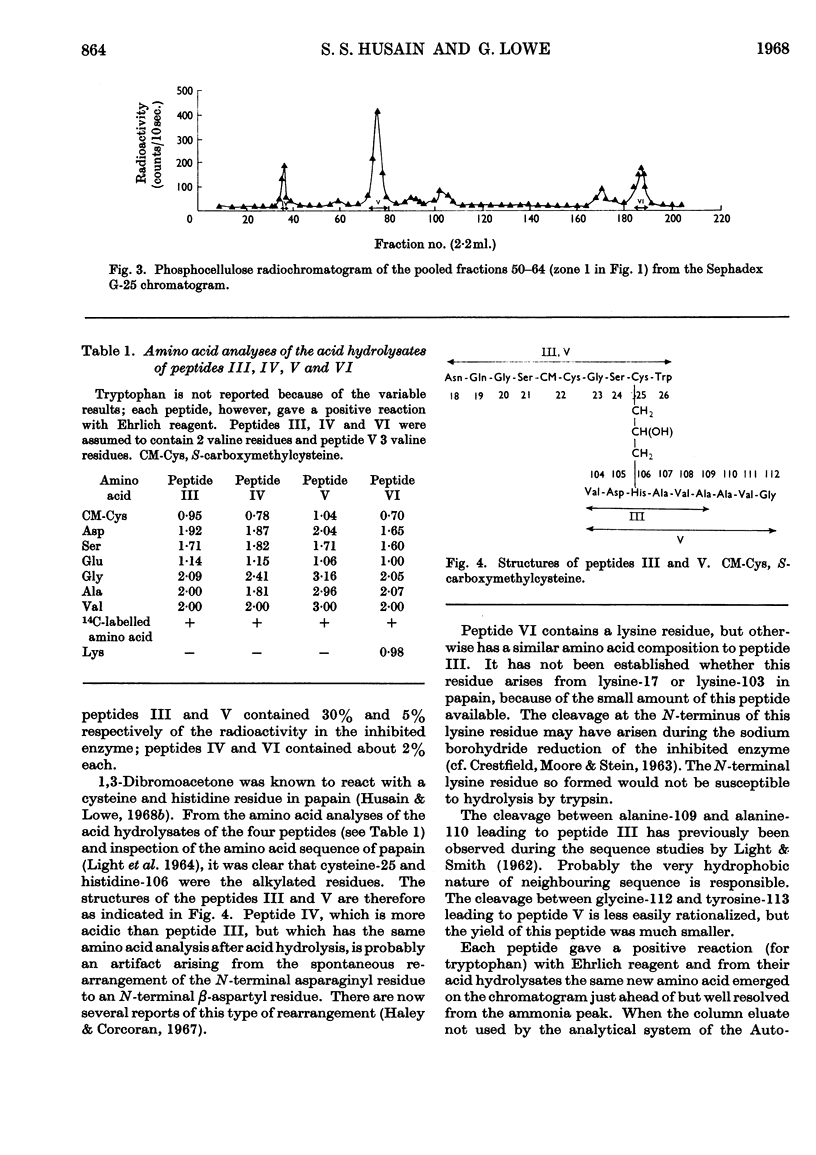
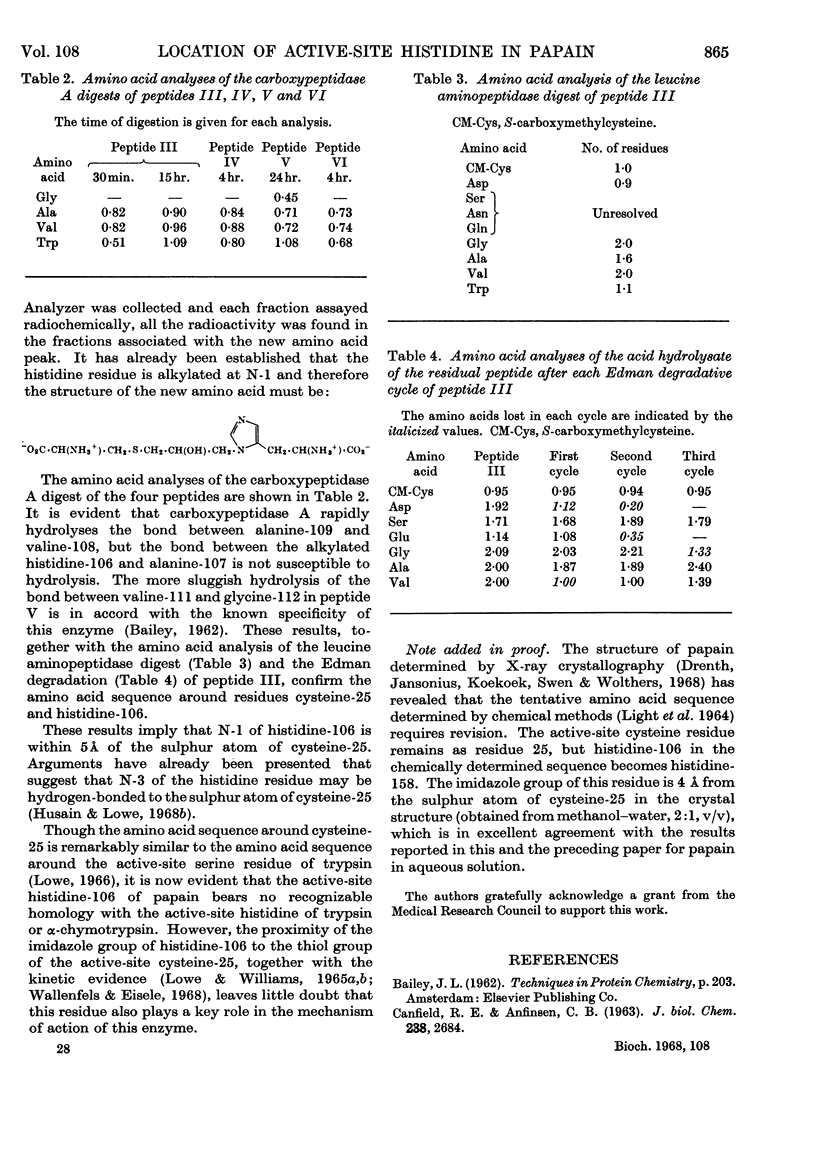
Papain that had been irreversibly inhibited with 1,3-dibromo[2-14C]acetone was reduced with sodium borohydride and carboxymethylated with iodoacetic acid. After digestion with trypsin and α-chymotrypsin the radioactive peptides were purified chromatographically. Their amino acid composition indicated that cysteine-25 and histidine-106 were cross-linked. Since cysteine-25 is known to be the active-site cysteine residue, histidine-106 must be the active-site histidine residue.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- CANFIELD R. E., ANFINSEN C. B. CHROMATOGRAPHY OF PEPSIN AND CHYMOTRYPSIN DIGESTS OF EGG WHITE LYSOZYME ON PHOSPHOCELLULOSE. J Biol Chem. 1963 Aug;238:2684–2690. [PubMed] [Google Scholar]
- CRESTFIELD A. M., MOORE S., STEIN W. H. The preparation and enzymatic hydrolysis of reduced and S-carboxymethylated proteins. J Biol Chem. 1963 Feb;238:622–627. [PubMed] [Google Scholar]
- Drenth J., Jansonius J. N., Koekoek R., Swen H. M., Wolthers B. G. Structure of papain. Nature. 1968 Jun 8;218(5145):929–932. doi: 10.1038/218929a0. [DOI] [PubMed] [Google Scholar]
- Haley E. E., Corcoran B. J. Beta-aspartyl peptide formation from an amino acid sequence in ribonuclease. Biochemistry. 1967 Sep;6(9):2668–2672. doi: 10.1021/bi00861a004. [DOI] [PubMed] [Google Scholar]
- Husain S. S., Lowe G. Evidence for histidine in the active site of papain. Biochem J. 1968 Aug;108(5):855–859. doi: 10.1042/bj1080855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KONIGSBERG W., HILL R. J. The structure of human hemoglobin. III. The sequence of amino acids in the tryptic peptides of the alpha chain. J Biol Chem. 1962 Aug;237:2547–2561. [PubMed] [Google Scholar]
- LIGHT A., FRATER R., KIMMEL J. R., SMITH E. L. CURRENT STATUS OF THE STRUCTURE OF PAPAIN: THE LINEAR SEQUENCE, ACTIVE SULFHYDRYL GROUP, AND THE DISULFIDE BRIDGES. Proc Natl Acad Sci U S A. 1964 Nov;52:1276–1283. doi: 10.1073/pnas.52.5.1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LIGHT A., SMITH E. L. Chymotryptic digest of papain. J Biol Chem. 1962 Aug;237:2537–2546. [PubMed] [Google Scholar]
- LOWE G., WILLIAMS A. A STUDY OF SOME THIOL ESTER HYDROLYSES AS MODELS FOR THE DEACYLATION STEP OF PAPAIN-CATALYSED HYDROLYSES. Biochem J. 1965 Jul;96:194–198. doi: 10.1042/bj0960194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWE G., WILLIAMS A. PAPAIN-CATALYSED HYDROLYSIS OF SOME HIPPURIC ESTERS. A NEW MECHANISM FOR PAPAIN-CATALYSED HYDROLYSIS. Biochem J. 1965 Jul;96:199–204. doi: 10.1042/bj0960199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- THOMSON A. R., MILES B. J. ION-EXCHANGE CHROMATOGRAPHY OF AMINO-ACIDS: IMPROVEMENTS IN THE SINGLE COLUMN SYSTEM. Nature. 1964 Aug 1;203:483–484. doi: 10.1038/203483a0. [DOI] [PubMed] [Google Scholar]
- Wallenfels K., Eisele B. Stereospecific alkylation with asymmetric reagents. Eur J Biochem. 1968 Jan;3(3):267–275. doi: 10.1111/j.1432-1033.1968.tb19526.x. [DOI] [PubMed] [Google Scholar]


