Abstract
Heart tissue is remarkably sensitive to oxygen deprivation. Although heart cells, like those of most tissues, rapidly adapt to anoxic conditions, relatively short periods of ischaemia and subsequent reperfusion lead to extensive tissue death during cardiac infarction. Heart tissue is not readily regenerated, and permanent heart damage is the result. Although mitochondria maintain normal heart function by providing virtually all of the heart's ATP, they are also implicated in the development of ischaemic damage. While mitochondria do provide some mechanisms that protect against ischaemic damage (such as an endogenous inhibitor of the F1Fo-ATPase and antioxidant enzymes), they also possess a range of elements that exacerbate it, including ROS (reactive oxygen species) generators, the mitochondrial permeability transition pore, and their ability to release apoptotic factors. This review considers the process of ischaemic damage from a mitochondrial viewpoint. It considers ischaemic changes in the inner membrane complexes I–V, and how this might affect formation of ROS and high-energy phosphate production/degradation. We discuss the contribution of various mitochondrial cation channels to ionic imbalances which seem to be a major cause of reperfusion injury. The different roles of the H+, Ca2+ and the various K+ channel transporters are considered, particularly the K+ATP (ATP-dependent K+) channels. A possible role for the mitochondrial permeability transition pore in ischaemic damage is assessed. Finally, we summarize the metabolic and pharmacological interventions that have been used to alleviate the effects of ischaemic injury, highlighting the value of these or related interventions in possible therapeutics.
Keywords: F1Fo-ATPase, ischaemia, mitochondria, reactive oxygen species (ROS), reperfusion
Abbreviations: [Ca2+]c, concentration of free cytoplasmic Ca2+; [Ca2+]m, concentration of intramitochondrial free Ca2+; HSP, heat-shock protein; IF1, natural inhibitor protein of the mitochondrial F1Fo-ATPase; iNOS, inducible nitric oxide synthase; K+ATP, ATP-dependent K+ channel; mK+ATP, mitochondrial K+ATP; MPT, mitochondrial permeability transition; [Na+]c, concentration of free cytoplasmic Na+; PDH, pyruvate dehydrogenase; PKC, protein kinase C; ROS, reactive oxygen species; SOD, superoxide dismutase; UCP, uncoupling protein; VDAC, voltage-dependent anion channel
INTRODUCTION
The heart is a pump that converts chemical energy into mechanical work. The power produced by cardiac muscle is generated almost entirely by the oxidation of carbon fuels with oxygen, and to a great extent the fuels are provided by coronary (myocardial) blood flow. Oxidative metabolism is the function of mitochondria within the cell, and thus the bulk of cardiac energy is supplied by oxidative phosphorylation within cardiac mitochondria.
Like many cells, when deprived of oxygen (anoxia), cardiac cells can maintain ATP levels by glycolytic ATP production, and can then revert smoothly to oxidative metabolism on reperfusion [1]. However, if blood flow is restricted, as in myocardial infarct, the cells accumulate glycolytic by-products (lactate, H+) in addition to suffering from oxygen deprivation [2]. This is a condition known as ischaemia and can damage cardiac cells irreversibly. Paradoxically, however, the major damage to ischaemic cells comes on the re-introduction of oxygen (reperfusion). During reperfusion, the cells typically undergo further contraction (hypercontracture) and membrane damage, followed by cell death [3,4].
It is widely acknowledged that ischaemia and reperfusion lead to mitochondrial, as well as cellular, damage in cardiac cells [5–9]. Furthermore, the phenomenological description of the mitochondrial changes that occur in ischaemia and reperfusion, together with pharmacological studies on agents that protect against such changes, suggest that mitochondrial dysfunction might be important as a major causative agent in tissue injury during ischaemia and reperfusion. The aim of the current review, therefore, is to discuss the roles of the mitochondrion in the pathology of ischaemia and reperfusion injury, and as a potential site for intervention to limit the damage. In particular, we discuss the roles of the electron transfer complexes, the F1Fo-ATP synthase, and mitochondrial ion channels, all of which normally mediate healthy mitochondrial function, as potential sites for promoting cell damage in this pathological situation. Other reviews have dealt with downstream events, such as the apoptotic pathway [10], the roles of ROS (reactive oxygen species) [11] and the long-term events of preconditioning [12].
NORMAL CARDIOMYOCYTE METABOLISM
Fatty acids provide the main energy source for the healthy heart, supplying 60–80% of its energy requirement. The energy balance arises from the oxidation of lactate and glucose in approximately equal proportions [13]. Substrate availability largely controls fuel use: when the concentration of non-esterified ‘free’ fatty acids is high in plasma, intra-mitochondrial NADH and acetyl-CoA levels rise, agents that inhibit pyruvate dehydrogenase and limit the use of 3-carbon fragments.
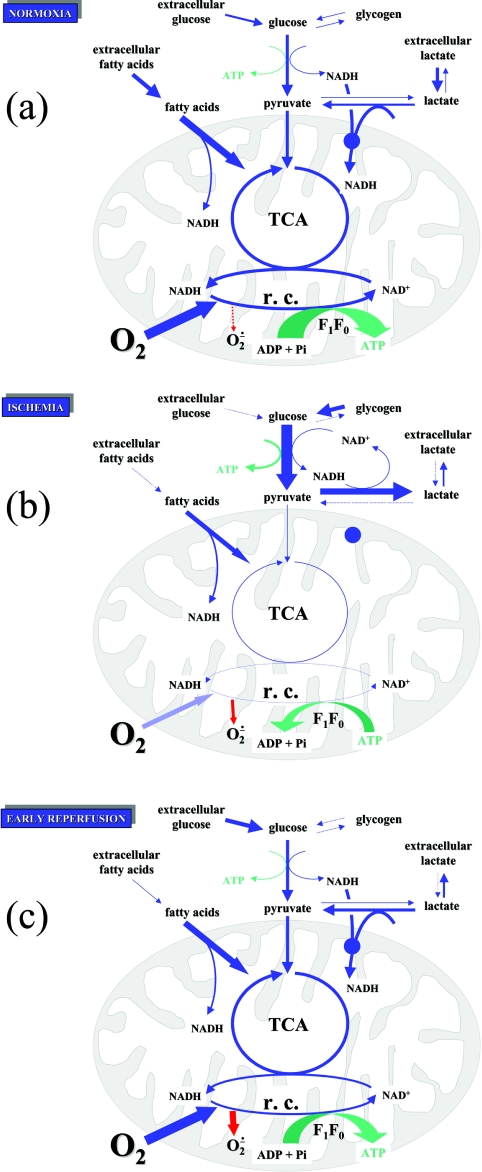
Over 90% of heart metabolism is aerobic, and the heart is a highly oxidative tissue, with an oxygen utilization rate of 60–150 mmol/min in humans [14]. Even at ambient oxygen concentrations, some of this oxygen is partially reduced to ROS, which are potentially damaging to the cells. Estimates from in vitro preparations put this value at 1–2%, although it may be lower in vivo. At these low levels, ROS are rapidly destroyed by mitochondrial and sarcoplasmic SODs (superoxide dismutases) and related enzymes [15]. The metabolism of normoxic heart cells is summarized in Figure 1(a).
Figure 1. Fuel utilization in heart mitochondria.
(a) Normoxia, where fatty acid oxidation provides the bulk of ATP. (b) Ischaemia, where glycolysis is the major ATP generator. (c) Early reperfusion, showing an increase in ROS production. Blue arrows indicate metabolic fluxes; green arrows indicate ATP production/hydrolysis; red arrows indicate ROS production; r.c., respiratory chain activity; O2− indicates all ROS. The thickness of arrows indicates the relative magnitudes of the fluxes involved. For a discussion, see the text.
Because of this high oxidative metabolism, heart cells have a high oxidative capacity. It has been estimated that 25–35% of total cardiomyocyte volume is occupied by mitochondria [16]. Two forms of mitochondria have been recognized in heart cells on the basis of position, and ease of extraction, and these are termed ‘interfibrillar’ and ‘subsarcolemmal’ [17]. It is thought that interfibrillar mitochondria provide most of the ATP for the contractile apparatus, although it is unclear whether there is a significant functional difference between these classes of mitochondria.
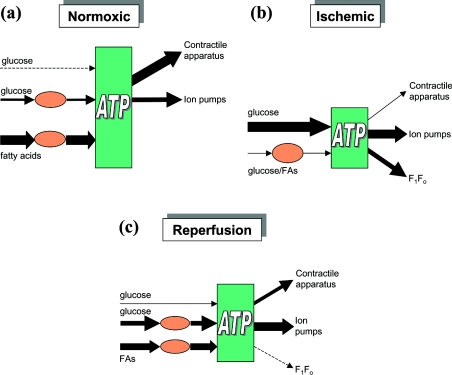
Use of ATP by the heart is linked closely to contraction. Approximately two-thirds of the ATP hydrolysed in the cardiomyocyte is used by the contractile apparatus (actomyosin) while the remaining third is used by pumps which maintain ion balance, in particular by the sarcoplasmic reticulum Ca2+-ATPase and the sarcolemmal Na+/K+-ATPase [18]. The ATP balance of normoxic cardiac cells is represented in Figure 2(a). The ATP used in these processes is regenerated by the mitochondrial F1Fo-ATP synthase using the energy liberated by the respiratory chain. Oxygen utilization by the heart is correlated with contraction [19]. The connecting link is the proton-electrochemical gradient across the mitochondrial membrane, comprising a concentration gradient of approx. 1 pH unit (60 mV) and an electrical potential (Δψ) of 140 mV (negative inside), giving a maximal ΔμH+ (electrochemical gradient of protons) of approx. 200 mV in the aerobic heart [20].
Figure 2. ATP balance in heart mitochondria.
The height of the green box indicates intracellular ATP concentrations under conditions of (a) normoxia, (b) ischaemia and (c) reperfusion. The sources and sinks for ATP are indicated as black arrows, with the relative fluxes indicated by the thickness of the arrows. The pink ovals indicate respiratory chain function. For a discussion see the text. FAs, fatty acids.
ATP in cardiac cells is in rapid equilibrium with creatine phosphate, which acts as a temporary store of ‘high-energy’ phosphate bonds. The enzyme involved is creatine kinase, which is present in the mitochondrial intermembrane space and in the sarcoplasm. There have been some claims that, aside from a store of energy, this system may also promote the apparent diffusion rate of ADP to the mitochondria and thus this organization is important for the efficient functioning of oxidative phosphorylation [21].
In the short-term, the contractile mechanism of the cell is controlled by the [Ca2+]c (concentration of free cytoplasmic Ca2+). Beats are characterized by [Ca2+]c transients, with [Ca2+]c rising from approx. 100 to 500 nM [22], due to release of Ca2+ from the sarcoplasmic reticulum and uptake from outside the cell via the slow sarcolemmal Ca2+ channels (both uniports). The low level of [Ca2+]c is then restored owing to the action of the sarcoplasmic Ca2+-ATPase and the sarcolemmal Na+/Ca2+ exchanger. These transients repeat with a period of approx. 1 s, depending on the organism.
[Ca2+]m (free intramitochondrial Ca2+) responds to [Ca2+]c via the inner membrane Ca2+ uniport [23], which mediates Ca2+ entry, and the mitochondrial Ca2+/Na + exchanger, which mediates Ca2+ exit. However, these act slowly relative to the above-mentioned transients, and the rapid changes in [Ca2+]c are damped, leaving a [Ca2+]m that reflects the time-averaged level of [Ca2+]c. These changes in [Ca2+]m are known to regulate dehydrogenases of the tricarboxylic acid cycle, and certainly play a role in regulating heart metabolism [24]. However, the large Ca2+ capacity of the mitochondria in heart cells has led to the suggestion that, in addition cardiac mitochondria might themselves be involved in controlling [Ca2+]c [24a].
METABOLIC CHANGES IN ISCHAEMIA AND REPERFUSION
Cardiac muscle is a highly aerobic tissue. As noted above, under normal conditions, it obtains virtually all its energy from oxidative metabolism. Consequently, restriction of the blood supply to cardiac muscle has serious pathological consequences, leading to cell death in the oxygen-depleted region (infarct).
During hypoxia or ischaemia, the supply of O2 to the respiratory chain fails. Non-esterified fatty acid levels rise, although probably as a result of lipid breakdown rather than the concomitant cessation of fatty acid oxidation [25,26]. The tricarboxylic acid cycle is blocked, and no energy is available from oxidative phosphorylation. This leads to an accumulation of cytoplasmic NADH, with the NADH/NAD+ ratio increasing severalfold. In anoxia, ATP levels can still be maintained by glycolysis [1], but in ischaemia this is accompanied by an accumulation of lactate and a decrease in cytoplasmic pH (5.5–6 after 30 min of ischaemia) [27–29], and glycolysis is also inhibited.
The energy charge of the cardiomyocyte during ischaemia has been investigated by a number of researchers, using both classical chemical analyses, and real time analyses using 31P NMR. Typically, creatine phosphate concentration falls precipitately (to less than 10% after 10 min of ischaemia), reflecting a sharp increase in free [ADP]. ATP levels fall rather more slowly, with 40–50% of [ATP] remaining after 30 min of ischaemia [30]. During ischaemia, the levels of total pyridine nucleotides seem to be roughly maintained, although there have been reports of significant loss (up to 30%) of total nucleotides from the cell [31–33]. Their redox state, however, changes markedly, with [NADH] increasing sharply (as described above) [34,35]. The cytoplasmic [NADPH], in contrast, declines by approx. 30%, resulting in a significant decrease in the NADPH/NADP+ ratio. While at first this may appear surprising, the fall in [NADPH] could be due to the action of glutathione reductase, which is particularly active under conditions of oxidative stress. In addition, Hwang et al. [36] suggest that a contributory effect may come from the activation of aldose reductase, a member of the aldo-keto reductase family that utilizes NADPH to reduce carbonyl compounds, including glucose, in the metabolism of polyols. Inhibition of this enzyme promotes glycolysis and improves recovery from ischaemia.
The ionic content of the sarcoplasm also changes markedly in ischaemia. Owing to low [ATP], the sarcolemmal Na+/K+-ATPase and the sarcoplasmic reticulum Ca2+-ATPase become ineffective, and cytoplasmic [Na+] and [Ca2+] rise [4]. Prolonged lack of mitochondrial oxidation will lead to abolition of ΔμH+, and this leads to (i) a decreased activity of the mitochondrial Ca2+ uniport, with decreased uptake of Ca2+ into mitochondria, and (ii) the operation of the ATP synthase, in reverse, as an ATPase. This ATPase activity is thought to contribute significantly (35–50%) to ATP loss in ischaemia [37], although this is disputed by some researchers [38]. The metabolism of ischaemic heart cells is summarized in Figure 1(b), and their ATP balance in Figure 2(b).
Over longer periods of ischaemia, DNA and protein synthesis are suppressed [39], although some specific proteins e.g. HSP (heat-shock protein)70, PKC (protein kinase C) ϵ, and iNOS (inducible nitric oxide synthase) may be induced [40,41] or repressed, e.g. ATPase ϵ [42]. On reperfusion, electron transfer and ATP synthesis restart, and the internal cytoplasmic pH is restored to 7.0 [2,43]. However, this leads in some way to a further deterioration of cell function. While ATP and creative phosphate levels recover to some extent, the myocytes undergo further shortening (hypercontracture) and membrane damage, followed by cell death [4], which can be measured in beating heart preparations by the release of the intracellular enzyme, lactate dehydrogenase, into the perfusate. From studies on simulated ischaemia in cultured cardiomyocytes, it appears that both ATP depletion and the low pH are required for cell death to occur. Cell death may be due to either necrosis or apoptosis, although, using markers such as DNA fragmentation, the latter appears to be the dominant contributor [44].
Many explanations for this deterioration are linked to abnormal Ca2+ movements. [Ca2+]c rises further, as indicated by hypercontracture [43] probably because of the reverse of the normal direction of the sarcolemmal Na+/Ca2+ exchanger. This increased cytoplasmic Ca2+, coupled with the restoration of mitochondrial membrane potential, leads to the accumulation of mitochondrial Ca2+ via the electrophoretic uniport, which has highly deleterious effects on mitochondrial function. The interaction between ions and mitochondrial function in ischaemia/reperfusion are discussed in more detail below. The metabolism of reperfused heart is summarized in Figure 1(c), and its ATP balance is shown in Figure 2(c).
Other researchers have emphasized the overproduction of ROS on reperfusion as a source of cell damage [45], and it is notable that approx. 50% of free protein SH groups disappear, presumably owing to interference with the glutathione redox system [35]. Although cytosolic NADPH can be involved in maintaining reduced glutathione (as described above), the balance may shift towards the production of ROS by cytosolic NADPH oxidase; blocking NADPH production by inhibiting glucose-6-phosphate dehydrogenase, as well as inhibiting its re-oxidation [by NADPH oxidase or NO (nitric oxide) synthase] is, unexpectedly, protective against reperfusion injury [46].
A rather surprising observation in these systems is that reperfusion injury can be decreased by pre-treating the heart with a brief ischaemic episode (3–5 min), followed by a short recovery period, before prolonged ischaemia. This is known as ‘preconditioning’ the heart. Protection is maintained over a period of several hours, between the preconditioning and the prolonged ischaemic insult and in the long-term it results from the activation of PKC, and induction of proteins within the heart [47]. The link between the initial brief ischaemia, in which the metabolic changes are as described above, and the pre-conditioned state are unclear. However, there is sufficient evidence to implicate mitochondria in this process, with ROS and the mK+ATP (mitochondrial K+ATP) channels as candidates in a triggering mechanism [48–50].
MITOCHONDRIAL OXIDATION
General observations
It is widely accepted that during prolonged ischaemia, the maximum capacity for respiratory chain oxidation is decreased, with the severity of damage depending on the length of exposure. This was first shown by Jennings et al. [51], who associated morphological changes in mitochondria, with the transition from reversible to irreversible injury of heart tissue, and with the loss of ability of mitochondria to oxidize NAD-linked substrates. These findings have been confirmed by many other researchers (for a review, see Piper et al. [8]). In accordance with the decreased respiratory capacity, oxidative phosphorylation was also found to be depressed in experimental models of ischaemia and reperfusion [52–54].
Not all studies, however, show a clearcut decrease in the activity of the respiratory chain itself. Lucas and Szweda [55] reported that in mitochondria isolated from perfused Langendorff rat hearts, respiration of NAD-linked substrates was decreased after ischaemia or reperfusion. However, they attributed the decrease, not to the modest decrease in activity of the respiratory complexes, but to inactivation of matrix 2-oxoglutarate dehydrogenase and the consequent decrease in NADH supply to the respiratory chain.
Several in vitro studies seem to indicate that there is no decrease in respiratory activity in mitochondria after exposure to 30 min of ischaemia [30,56,57], and NMR studies of flux indicate that any damage is not sufficient to slow down coupled electron flow [58]. Indeed, recent work indicates that rates of coupled [ADP-stimulated-state III] and uncoupled 2-oxogluatarate and succinate oxidation increase following 30 min of ischaemia and 30 min of subsequent reperfusion [59]. These enhancements were attributed to increased mitochondrial matrix volume.
Minners et al. [60] reported increased rates of oxidation of endogenous substrates in various cell types after ischaemia/reoxgenation. This seems to be attributable to increased proton leakage in the mitochondria after an ischaemic insult. Taniguchi et al. [61] reported that at state IV (maximal attainable) membrane potential was approx. 10 mV lower in mitochondria isolated from hearts that were exposed to 30 min of ischaemia followed by 60 min of reperfusion, than in those isolated from control hearts (175 mV compared with 185 mV), and O2 consumption rate (state IV respiration) was correspondingly greater in the former, 128 nmol O2/mg per min, as compared with 77 nmol O2/mg per min in the latter. An increased proton leak in ischaemia-damaged mitochondria was also deduced by Borutaite et al. [62] using a detailed kinetic analysis of respiration rates. Thus, it has been shown that after ischaemia/reperfusion in heart, respiration may fall, rise or remain the same. This is explicable in terms of the balance between three possibly limiting factors: (i) activity of the respiratory chain complexes themselves, (ii) proton leak at the mitochondrial inner membrane, and (iii) supply of respiratory substrates, as discussed below.
Ischaemia/reperfusion does cause some damage to respiratory chain complexes, but as these complexes are normally present in excess (having a low flux control coefficient), this damage has little effect on normal respiratory rates. Indeed, respiration rates may be observed to rise owing to an increased proton permeability of the inner-mitochondrial membrane (decreased respiratory control). This rise, however, will be dependent on an ample supply of oxidizable substrate, which may, in some conditions, be restricted and itself limit the respiratory rate observed.
Further complications in identifying a particular source of damage arise from the variety of assay methods used. Oxygen uptake, using a Clark electrode, has typically been measured either in mitochondria isolated from ischaemic heart or in skinned muscle fibres [10,63]. In the case of isolated mitochondria, the preparation may not represent the population of mitochondria in the original tissue. First, mitochondria may change, particularly in substrate and ion content, during isolation. Secondly, Jennings et al. [51] have shown that mitochondria isolated from ischaemic heart are more fragile that those isolated from normal heart. Thirdly, typical mechanical isolation procedures appear to yield largely subsarcolemmal mitochondria, while the interfibrillar mitochondria, which provide most of the energy for the contractile apparatus, are under-represented [64]. In skinned fibres, on the other hand, there may be problems with accessibility of substrates to the mitochondria [65], and respiration rates may be limited by diffusion rather than by the intrinsic activities of the enzyme involved.
In an alternative approach, Ozcan et al. [66] attempted to mimic conditions of ischaemia and reperfusion on a sample of mitochondria isolated from the heart. These conditions led to a sharp decrease of ADP-induced respiration after reperfusion, larger than is known to occur within the tissue, owing to high levels of ROS being produced at reperfusion. These observations highlight the problems in carrying out experiments with minimal in vitro preparations. The observations show how respiration and ATP synthesis might be affected without the endogenous mechanisms which protect mitochondrial function in the cell; they give little indication of what actually does occur within the cell milieu.
NADH dehydrogenase (complex I)
Many researchers have identified complex I as a major site of damage to the respiratory chain in ischaemia [56,67–69]. They observed a reduction in oxidation rate for NADH-linked substrates by up to 60%. Typically, oxidation rates with succinate were unchanged, suggesting that damage was restricted to complex I. Damage may increase on reperfusion [70].
Direct measurements of complex I activity in dog-heart mitochondria were made after ischaemia by total ligation of the left anterior descending coronary artery [67,71,72]. Activity was markedly decreased after 20–30 min of ischaemia. This may be due to effect of low pH on the enzyme, since Rouslin [73] observed that in vitro exposure of the enzyme to pH 6 for 1 min reduced enzyme activity by 40% in the absence of ATP. However, the relevance of this observation to the in vivo situation is unclear, since 1–2 mM ATP protected complex I against this inactivation through preservation of Δψ and by a direct effect of the nucleotide upon the complex.
Similar effects are seen in rat heart. Veitch et al. [68] found a significant reduction in complex I activity in perfused rat hearts exposed to 20 min of global ischaemia, and Cairns et al. [69] showed that this damage was exacerbated by reperfusion. Maklashina et al. [74] reported a 40% reduction in complex I activity upon exposure of cardiomyocytes to 30 min of nitrogen perfusion (hypoxia), and that this change was reversed on reperfusion. In contrast, Hardy et al. [56], found a specific decrease in complex I activity only upon reperfusion subsequent to hypoxia, both in mitochondria isolated from hypoxic-perfused hearts and in isolated cardiomyocytes subjected to a similar protocol of hypoxia/reperfusion.
The cause of complex I inhibition is unknown. Paradies et al. [75] associated the decrease in complex I activity under conditions of oxidative stress with the destruction of mitochondrial cardiolipin by ROS. Other researchers have suggested that NO might be the causative agent, possibly via peroxynitrite intermediates [76,77], since inhibitors of iNOS prevent the inactivation. This view is supported by Jekabsone et al. [78], who showed that complex I activity declines in isolated mitochondria exposed to NO, and that this decline is prevented by SOD. Thus either ROS or NO (or both) at the concentrations reachable during ischaemia and reperfusion could inactivate the enzyme directly, or via an effect on cardiolipin.
Thus it seems likely that the oxidative capacity of complex I does fall during ischaemia and especially during reperfusion. While some researchers have shown that NADH dehydrogenase can have a significant flux control coefficient (≈0.2) in isolated heart mitochondria (at limiting pyruvate/Ca2+ concentrations) [78a,78b], studies indicate that any such fall in complex I activity does not limit respiration in vivo. Sako et al. [79], using NMR measurements of ATP synthesis in isolated perfused hearts, and using pyruvate as sole energy source, found that the rate of myocardial oxygen consumption was, higher in reperfused ischaemic than in control hearts. Neubauer et al. [80] also observed high oxygen consumption rates following reperfusion after prolonged ischaemia, and recent work by Lim et al. [59], reporting studies on mitochondria rapidly isolated from perfused Langerdorff-rat hearts, showed an increase in respiration rate with both NADH-linked and succinate substrates. These researchers explained the increase in complex I turnover as a defect in coupling energy production to utilization in ischaemic/reperfused hearts, rather than as a feature of complex I itself.
Succinate dehydrogenase (complex II) and cytochrome c reductase (complex III)
Both complex II and complex III seem to be relatively resistant to ischaemia and reperfusion. Rouslin [67], found that complex III activity did decline in ischaemic dog heart, but more slowly than complex I activity. Its decrease paralleled that of succinate-supported oxygen uptake, implying that complex II was not affected by either ischaemia or reperfusion. Similar results were reported by Piper et al. [81] and by Veitch et al. [68] in rats. Veitch showed that 5 min of reperfusion could cause significant (34%) loss of complex III activity after 60 min of ischaemia, and this was associated with slightly decreased content of two components of complex III, cytochromes b and c1. Hoppel et al. [82] confirmed loss of complex III activity in both subsarcolemmal and interfibrillar mitochondria, but showed that the decreased activity proceeded via impairment of the iron–sulphur subunit without the loss of the apoprotein. However Petrosillo et al. [83] showed that loss of complex III activity paralleled the loss of cardiolipin from mitochondria and proposed that a substantial contribution to respiratory inhibition should be ascribed to cardiolipin degradation owing to oxidative attack by oxygen free radicals. Guidarelli and Cantoni [84] showed that peroxynitrite caused inhibition of complex III activity in cultured cells, and this too may contribute.
The above researchers do not suggest any significant effect of ischaemia/reperfusion directly on the succinate dehydrogenase enzyme, and indeed Lim et al. [59] showed that this enzyme was not affected. However, Abe et al. [76] reported that rat hearts exposed to ischaemia (20 min) and subsequent reperfusion (20 min) had a significantly decreased complex II activity, which was less than, but in addition to, a decrease in complex I activity. This decrease was attributed to NO and ROS, generated within the myocytes, inactivating the enzyme.
Cytochrome c oxidase (complex IV)
The situation regarding complex IV is more complicated. Several reports indicate it to be virtually unchanged by ischaemia [57,67,68,72,81]. Veitch et al. [68] showed that the cytochrome aa3 content of complex IV was unaffected by ischaemia for up to 60 min, and a further 30 min of reperfusion (although approx. 25% seemed to be lost during 60 min of ischaemia and 60 min of reperfusion). Borutaite et al. [85] and Lesnefsky et al. [86] showed that the maximal catalytic capacity of cytochrome oxidase, and its apparent Km for oxygen, was unchanged after an ischaemic insult.
However, there did appear to be reduced electron flow through complex IV both in permeabilized ischaemic tissue and mitochondria isolated from ischaemic heart [85,86]. This may be attributable to the loss of cytochrome c, which mediates electron transfer between complexes III and IV, from the intermembrane space in subsarcolemmal mitochondria during ischaemia. Borutaite et al. [85] claimed that cytochrome c oxidase activity decreased by nearly 30% during ischaemia (from 0.156 to 0.111 nmol/mg of protein) and that full respiration rates are restored by external addition of cytochrome c. These authors also showed that cytochrome c is found in the perfusate of hearts, following ischaemia/reperfusion [85], suggesting it can indeed be lost from mitochondria in pathological situations. In contrast, Veitch et al. [68] claim that cytochrome c content of mitochondria is not significantly changed after ischaemia.
Other researchers suggest complex IV may be damaged more directly [85,86]. Paradies et al. [87] showed that, even though cytochrome aa3 levels did not change, the catalytic capacity of cytochrome oxidase was reduced by 25% after 25 min of ischaemia, and fell further, to 51%, after another 15 min of reperfusion. Other authors [69] observed that cardiac ischaemia and early reperfusion, induced an increased cytochrome aa-3(ox)/cytochrome aa3 (red) ratio, indicating inhibition of electron input to this complex. Surprisingly, they showed that the correct redox balance could be restored by post-ischaemic administration of succinate, rather than by cytochrome c. Most damage sustained directly by complex IV seems to occur after ischaemia, and during reperfusion. Possible mechanisms include loss/oxidation of cardiolipin [87,88], or formation of 4-hydroxynonenal adducts [89]. Approx. 50% of complex IV activity might be lost in this way.
The effect of inhibition of cytochrome oxidase, either by direct damage or by loss of cytochrome c, might not be expected to have a major effect on respiratory rates in vivo. This enzyme is normally present in excess of respiratory demand and has a very low flux control coefficient [78a,78b], thus limited inhibition would seem unlikely to be a factor in decreasing oxidative phosphorylation in post-ischaemic conditions. However, Gnaiger and co-workers [90,91] have pointed out that assays for cytochrome oxidase activity are normally carried out under conditions far from physiological. At the low oxygen tension which might prevail in vivo, cytochrome oxidase activity may in fact become a rate-limiting factor, as has been demonstrated by Kunz et al. [92]. Thus the restriction on the rate of respiration caused by loss of cytochrome oxidase activity, either directly or by cytochrome c depletion, may represent a pathological effect.
In this discussion of the effects of ischaemia/reperfusion on the respiratory chain, the reader will note that there is considerable variation between observations from different groups. Factors involved appear to include the species of animal involved, its age, the degree of anoxia in different models of ischaemia and even the different techniques used to collect data. This implies that the relative importance of several variables may change between preparations. Nonetheless, a consensus would indicate that exposing hearts to 15–60 min of ischaemia leads to a modestly impaired electron transfer chain, with rates reduced by approx. 30% relative to the control value. Complex I appears to be damaged, and there is some restriction of electron flow through complex IV, possibly because of loss of cytochrome c. During reperfusion, further damage to electron transfer components occurs, in particular to complex IV, possibly due to the action of ROS (as described below), and a ‘vicious cycle’ in which further inhibition leads to further ROS production, and so on, is a possible cause of irreversible damage to mitochondria.
OXYGEN FREE RADICALS: ROS IN ISCHAEMIA AND REPERFUSION
Cardiomyocytes, like many other types of cell, produce ROS. It has been estimated that, under normal physiological conditions, 1–2% of electron flow through the mitochondrial respiratory chain gives rise to ROS [91,93]. It might be expected that ischaemia, caused by low partial pressure of O2, would decrease ROS production, but this is paradoxically increased [30,94,95], with a further increase occurring on reperfusion [30,96]. Cell damage can occur through mechanisms involving lipid peroxidation, covalent modification of protein (particularly on -SH groups) and mitochondrial DNA oxidation (for a review, see [97]). Certain protective responses are induced by low levels of ROS, notably gene transcription and cell proliferation [98–100], but higher levels ultimately lead to cell death by apoptosis or necrosis [101,102].
Under normal conditions, scavenging mechanisms operate swiftly to remove excess ROS. Superoxide (O2−) is removed by SOD in cytosol (Cu/Zn-SOD) and in mitochondria (Mn-SOD) [103], and the resultant H2O2 is removed by catalase, glutathione peroxidase and peroxiredoxin [104,104a,104b]. However, elevated levels of ROS can swamp the protective mechanisms of the cell, and are a significant cause of ischaemic/reperfusion damage. The importance of ROS in inducing ischaemic damage is shown by a protective effect in perfused hearts of transgenic mice overexpressing Mn-SOD [105] and by a study showing that post-ischaemic recovery of contractile function is impaired in Mn-SOD-knockout mice [106]. Direct measurements of SOD levels have, unfortunately, yielded equivocal results, with Ueta et al. [107] indicating a decrease in SOD in ischaemia, but more recent work indicating increased activity associated with mitochondrial protection and a reduction in apoptosis, possibly through a mechanism involving HSP72 [108].
In general, ROS may arise from the operation of cytoplasmic xanthine oxidase. In heart, however, mitochondria probably constitute the principal source of ROS, since the respiratory chain deals with most of the electrons potentially capable of reducing O2 [95,109]. Redox components of the respiratory chain, particularly impaired complexes I and III in their reduced state, have been shown to produce ROS [110,111], possibly via ubisemiquinone radicals [112]. Complex I, in particular, is impaired during ischaemia/reperfusion (as described above), and may be considered as the source of the damaging radicals, although experiments using the electron transfer inhibitors amytal and myxathiazole suggest complex III as a major site of ROS production during ischaemia [95]. Recent studies indicate that complex II may also produce ROS [112a], particularly if certain mutations are present in regions of the CoQ (ubiquinone) or the FAD-binding sites [113,114].
In conclusion, cardiac ischaemia induces increased generation of ROS, and subsequent reperfusion can result in toxic ROS overproduction that possibly contributes to irreversible damage of mitochondrial function and consequent impaired recovery of physiological function and cell death.
Nitric oxide
NO is an endogenous mediator of several important physiological processes and it is involved in a number of protective mechanisms in cells [115]. However, overproduction of NO can occur and this can lead to cytotoxicity. The balance between protective and deleterious effects of NO has led to difficulties in assessing its role(s) in ischaemia/reperfusion. NO levels do rise during ischaemia and reperfusion and, under these conditions, NO can interfere with mitochondrial functions [76]. Thus NO can induce cell death by necrosis through inhibition of mitochondrial respiration [116,117], or trigger apoptosis mediated by the mitochondrial permeability transition and by cytochrome c release [118].
NO certainly inhibits respiration in mitochondria. It has a direct effect on cytochrome c oxidase, binding to the oxygen-binding site and hence inhibiting respiration. It is unlikely, however, that this type of inhibition is greatly damaging in ischaemia/reperfusion, since it is competitive and readily reversed when the inhibited enzyme is exposed to oxygen [119]. A more likely source of respiratory chain inhibition might come indirectly, by its forming covalent, peroxynitrite or S-nitrosothiol derivatives with proteins, and particularly with components of complex I or III [77,84]. Ubiquinol, which can reduce peroxynitrite, is believed to protect mitochondria against such damage to some extent [120], but with increasing amounts of peroxynitrite, such modifications are likely to arise, and will be irreversible in the physiological milieu.
Whether these changes lead to apoptosis, or other cell death mechanisms, is, however, controversial [121,122]. Several other researchers have demonstrated protective effects of NO in ischaemia and reperfusion. Rakhit et al. [123] found that rat ventricular myocytes in culture were protected by NO donors when the cells were exposed to 1 h of simulated ischaemia and 1 h of reperfusion. Protective effects have also been demonstrated in perfused mouse heart subjected to ischaemia and reperfusion [124]. A protective role for NO against loss of mitochondrial Δψ and apoptosis was also shown by Beltran et al. [125], albeit in the largely glycolytic Jurkat cells.
The main site of NO synthesis in cells seems to be extra-mitochondrial, as the activity of mitochondrial NO synthase is low. Thus intramitochondrial NO presumably changes in response to cytoplasmic NO [126]. These levels appear to be buffered by the formation of nitrosyl–haem complexes which form in significant amounts in the ischaemic heart [127]. However, it has also been reported that the mitochondrial NOS is functionally upregulated (2-fold increase) after hypoxia in animal models [128], implying a role for the mitochondrial enzyme in tissue protection.
ATP SYNTHASE
Heart muscle is essentially an aerobic tissue and, under normal circumstances, over 90% of its ATP is made by oxidative phosphorylation, and hence by the F1Fo-ATP synthase. This is a complex enzyme, comprising a transmembrane H+ channel (Fo), and a nucleotide-binding extrinsic sector (F1). Two inhibitory proteins, the H+/Δψ-dependent IF1 (natural inhibitor protein of the mitochondrial F1Fo-ATPase), and the Ca2+-binding ATPase-inhibitor protein, are believed to associate with the ATP synthase (for reviews, see [129,130]). Ca2+-activation of the ATP synthase has been demonstrated with increased work load/respiratory rate in rat and pig heart [1,131], although the quantitative importance of this effect in normal function is uncertain [132,133].
In the context of the current review, however, an important feature of the F1Fo-ATP synthase is its reversibility. The synthase can hydrolyse ATP and use the energy released to pump protons out of the mitochondrial matrix, increasing Δψ. Using oligomycin as a specific inhibitor of the ATP synthase, it has been shown that, in ischaemia, a considerable fraction (30–50%) of the decline in cytoplasmic high energy phosphates is due to hydrolysis by the ATP synthase working in reverse [37,67]. Although it is possible that limited reversal of the ATP synthase activity may have some useful (but unknown) role in vivo, this precipitous loss of, for example, over 95% of creatine phosphate within 5 min is clearly deleterious to the cell.
The tendency of the F1Fo-ATP synthase to reverse during ischaemia thus appears to be an inescapable consequence of the thermodynamics of the system, and in the absence of the energy-yielding reactions of electron transfer, ATP synthesis is unfavourable and hydrolysis is favoured. However, there does appear to be some endogenous regulatory mechanism restricting hydrolysis by the ATP synthase. Several groups have shown that the rate of ATP hydrolysis by mitochondria from ischaemic hearts or cardiomyocytes is decreased below that of mitochondria from normoxic hearts in dogs [134–137] and humans [132]. There has been some dispute as to whether the same inhibition was observed in rat heart, but this seems to be the case [1,27]. A study by Ylitalo et al. [132] shows that failure by some researchers to observe the effect in rat was probably due to lack of pH control in the isolation media.
Typically, ischaemia-induced inhibition of F1Fo-ATPase activity in vitro is approx. 50% of maximal activity (although the procedures used, involving extracting mitochondria from the heart muscle, may underestimate inhibition in vivo), with inhibition developing over 2–5 min. The kinetics and pH dependence of the effect suggests that inhibition is due to combination of the F1Fo-ATPase with the IF1 protein.
Inhibition of wasteful ATP hydrolysis during ischaemia might be assumed to have a beneficial effect on the heart. During reperfusion, when the ATP synthase should be working to synthesize ATP, however, inhibition of this enzyme would presumably delay recovery. This is explicable in terms of the properties of IF1, which is known to bind to F1Fo at low pH and low Δψ (such as occur in ischaemia), but to be released at high pH and high Δψ (such as occurs during electron transfer) [138], acting, effectively, as a one-way valve. Thus, during reperfusion, we would expect F1Fo to be re-activated. This reversibility of inhibition, again on a 2–5 min time scale, has been demonstrated in dog, rat and goat hearts [37,129,132,139,140]. Thus the active/inactive transitions of the F1Fo-ATP synthase do not contribute directly to the adverse effects of reperfusion, although it is likely that ATP hydrolysis by the synthase again becomes significant if the mitochondria undergo a permeability transition, and consequent depolarization, after reperfusion (see below).
Preconditioning is a phenomenon in which a brief period of ischaemia will protect the heart against subsequent prolonged periods of ischaemia/reperfusion. Preconditioned hearts show decreased tissue damage, and increased recovery of high-energy phosphates and contractile activity during reperfusion after an ischaemic insult. However, it is difficult to relate this unambiguously to changes in F1Fo activity. There are conflicting observations even as to the fate of ATP in pre-conditioned hearts. Jennings and co-workers [136,141] and Hassinen et al. [142] show a decrease in ischaemic ATP loss after preconditioning in dog and rat heart respectively, while Green et al. [143] show a greater loss in rat heart.
In view of the time scale of changes in ATP synthase capacity, which occur over a 2–5 min time period, it seems unlikely that the F1Fo-ATP synthase, or IF1, are involved in the phenomenon of preconditioning. This view is supported by the work of Jennings and co-workers [136] and Green et al. [143]. However, Hassinen and co-workers found that the drop in the hydrolytic capacity of the F1Fo-ATPase induced by ischaemia was greater after preconditioning than in untreated rat heart [27,144], and other researchers [136,140] have confirmed that, at least, a transient inhibition of F1Fo is induced by preconditioning. Although the reason for this increased inhibition is uncertain, it may indicate that the F1Fo-ATP synthase does have some role to play in the preconditioning phenomenon.
MITOCHONDRIAL MEMBRANE POTENTIAL AND ION CHANNELS
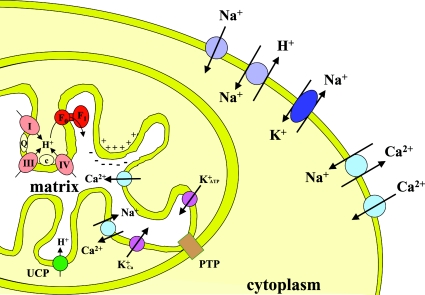
The inner-mitochondrial membrane has a limited, and highly selective, permeability to ions. There exists an extensive family of anion transporters that are largely antiports (Pi/OH−, ATP/ADP, malate/aspartate exchanger, etc.) [145], which are involved with substrate import. It is probable that these, in their normal form, do not contribute specifically to ischaemic/reperfusion damage, although the ability of the ATP/ADP translocator, when modified, to participate in the mitochondrial permeability pore may be important in promoting cell death in the longer term (as described below). It is in the area of cation transport (H+, Ca2+, K+) that most attention has been focused.
Since the energy released by the respiratory chain is stored as an ΔμH+, the permeability of the mitochondrial membrane to H+ is necessarily limited. Below dielectric breakdown, H+ can enter the mitochondrion only via the Fo channel of the F1Fo-ATP synthase or via controllable UCPs (uncoupling proteins) [146]. The other main routes for cation entry are the Ca2+ uniport (which is opposed by an Na+/Ca2+ exchanger), the low capacity K+ATP channels and the recently described K+Ca channel [147] (Figure 3).
Figure 3. Cation transporters in heart cells.
Physiological fluxes of ions are indicated by the arrows. Circles refer either to uniports or antiports. Ellipses indicate ATP-coupled transport systems. Inner membrane respiratory chain complexes are denoted I, III and IV; c, cytochrome c oxidase; Q, ubiquinone; PTP, permeability transition pore.
H+ movements
On a simple model, it is expected that, during ischaemia (as mitochondrial electron transfer is abolished) ΔμH+ will fall rapidly. It should then rise during reperfusion, when electron transfer is restored. While this seems in principle to be the case, investigations reveal a greater complexity. First, in ischaemia, a mitochondrial Δψ is maintained for some minutes [148] due to the hydrolysis of cytoplasmic ATP by the F1Fo-ATP synthase acting in reverse as an ATP driven pump [37,67]. This hydrolysis will decrease cellular ATP levels, and is gradually switched off by a regulatory element at the level of the ATP synthase (as described above). It is unclear whether the maintenance of Δψ is a physiologically useful phenomenon (e.g. in maintaining ion distributions), or whether this ATP hydrolysis is an unavoidable pathological process.
Conversely, during reperfusion, we should expect ΔμH+ to recover rapidly. Again, however, moderating factors seem to be operating. A number of researchers have reported that, after reperfusion, Δψ is depressed from its control value, and that mitochondria have an increased proton leak. This was originally demonstrated by analysis of flow force relationships in isolated heart mitochondria after ischaemia (and/or reperfusion) [56,85,149,150]. However, it also seems to be the case in heart itself, as indicated by an increased rate of O2 utilization relative to work output (i.e. a decreased cardiac efficiency) on reperfusion [151]. It is possible, again, that this change represents either unavoidable mitochondrial damage during ischaemia/reperfusion or the preferential use of oxidative energy to restore ion concentrations. However, a number of researchers have suggested that the depression of Δψ may play a protective role during early reperfusion, limiting the electrophoretic influx of Ca2+ and/or decreasing the production of ROS by facilitating electron transfer (see below). Agents that promote the leakage of protons into mitochondria, such as uncouplers [152,153], or transfection with UCPs [154,155] have been shown to protect against ischaemia/reperfusion injury.
Ca2+ movements
The Ca2+ uniport acts electrophoretically, and is thus dependent on the Δψ component of the electrochemical potential. Under normal conditions, [Ca2+]c in the sarcoplasm, like [H+]c, is approx. 10−7 M. Under these conditions, the low affinity of the Ca2+ uniport for Ca2+ (and the operation of the Na+/Ca2+ exchanger) means that [Ca2+]m is slightly lower than [Ca2+]c [156]. Under normal physiological conditions, [Ca2+]c would rise with increasing work load, and [Ca2+]m would mirror this change. The rise in [Ca2+]m leads to an increase in activity of the dehydrogenases of the tricarboxylic acid cycle [157] and increased oxidation rates. In heart muscle, where mitochondria make up 25–35% of cell volume, it is also believed that mitochondria can play an active role in modulating [Ca2+]c [158].
Although total myocardial Ca2+ does not change during ischaemia [63], [Ca2+]c increases significantly as ATP-driven pumps (notably the sarcoplasmic Ca2+ pump) become less active. [Ca2+]m will also tend to rise, but this rise is limited by the lowered mitochondrial membrane potential. The major increase in [Ca2+]m occurs on reperfusion, when the mitochondrial membrane repolarizes [159]. A study in guinea-pig heart by Varadarajan et al. [160] showed that during ischaemia, [Ca2+]m rises only by approx. 50%, whereas the increase was 4–5-fold during reperfusion. They also showed that this high level was maintained for up to 1 h during perfusion, even though [Ca2+]c returned to normal within 10 min, suggesting that ischaemia had induced a chronic problem in mitochondrial Ca2+ handling.
It seems likely that these abnormal Ca2+ distributions are a major contributor to ischaemia/reperfusion cell damage. A number of researchers have shown that if Ca2+ entry into mitochondria is blocked, cell damage (or infarct size, in heart preparations) is much reduced. Ruthenium Red, which blocks the mitochondrial Ca2+ uniport, has been shown to have a protective effect [161,162], and other, less direct effectors that have a protective effect, also oppose the mitochondrial Ca2+ uptake. These effectors include inhibitors of the sarcolemmal Na+/H+ exchanger [150,163], uncouplers [153] and low [Ca2+] media [164]. Arieli et al. [165] have recently shown that cardiac mitochondria from male rats are more susceptible to Ca2+-induced depolarization than females, and suggest that this may account for the higher susceptibility of males to ischaemic heart damage.
K+ movements
In recent years, there has been considerable interest in the mK+ATP in the context of ischaemia/reperfusion. Such channels allow K+ entry and are blocked by ATP. Thus, in the absence of ATP (e.g. in ischaemia), mK+ATP should open, leading to a decrease in Δψ and an interior alkalinization of the mitochondrion (ΔμH+ remaining constant). There is evidence for a K+ATP channel in heart mitochondria [166], and its molecular composition, although not yet clear, is different from other cytomembrane K+ATP channels [167]. Its role, however, is controversial, as it appears to have a low capacity, and it has been difficult to demonstrate significant changes in Δψ whether it is open or blocked [168].
Pharmacological evidence indicates that the mK+ATP channel in heart exerts its effects on the phenomenon of preconditioning. This is a protective effect, observed as a result of subjecting a heart to a brief period of hypoxia, before the major ischaemic insult. A number of researchers have reported that diazoxide (which opens the mK+ATP channel) will induce the same protection as preconditioning, while 5-hydroxydecanoate, (which blocks the channel), will abolish its protective effect [169–171]. It is proposed that these effects are due to the brief release of ROS, induced by some unknown mechanism, on opening the K+ATP channel, and this in turn triggers a variety of long-term protective responses, which are outside the scope of this review. ROS production is indeed induced in intact heart cells by diazoxide [172], although paradoxically this agent lowers ROS in mitochondrial preparations [173]. This work has recently been reviewed by O’Rourke [174].
Other researchers have questioned the role, or even the existence, of functioning K+ATP channels in heart mitochondria. Askenasy et al. [175] could not demonstrate an effect of K+ATP channel blockers in rat heart. Diazoxide and hydroxydecanoate have been shown to inhibit or activate mitochondrial electron transfer respectively [176,177], and it could be that these agents exert their effects on preconditioning independently of any effect on the mK+ATP channel. Diazoxide is also known to promote IF1-binding to F1, which could itself be cardioprotective [178]. Dos Santos et al. [179], and Ganote and Armstrong [180], have emphasized possible roles of the mK+ATP channel in maintaining mitochondrial volume in cells, while Das et al. [181] were unable to demonstrate any effects of a putative K+ATP channel on the volume of isolated rat heart mitochondria. As yet, therefore, any role of the K+ATP channel in ischaemia/reperfusion injury should be viewed with caution, especially in view of the recent report of a mitochondrial K+Ca channel, which might link K+ movements with Ca2+ levels in heart cells [182] (Figure 3).
Na+ movements
Abnormal levels of other ions are also known to contribute to ischaemia/reperfusion damage. The best known, perhaps, is Na+. NMR and probe studies indicate that intracellular [Na+] roughly doubles in perfused heart during a 20–30 min period of ischaemia, with a further increase of 50–150% during a 30–60 min period of reperfusion [163,183,184]. Reperfusion, in fact, induces periodic Ca2+ transients which lead to hypercontracture and mechanical damage to the myocyte [4]. Inhibitor studies indicate that the bulk of the Na+ ions enter the cell through the sarcolemmal Na+/H+ exchanger [150,185], probably as a response to lowered internal pH during ischaemia and reperfusion [186], although there is some contribution from fast Na+ channels, and a lowered Na+/K+ pump activity [173]. During reperfusion, Na+ continues to move into the cell (through both the Na+/H+ exchanger and the Na+/HCO3− symport) to relieve the acid load that has built up [187].
Although this increased [Na+]c (concentration of cytoplasmic free Na+) is damaging to heart cells, its action is not simple. Mitochondria may be affected directly by Na+ acting on mitochondrial swelling and inducing abnormal mitochondrial permeability, and such effects of Na+ can indeed be demonstrated in isolated mitochondria [163]. However, the observed damage is more likely due to the indirect effects of [Na+]c on both proton and Ca2+ fluxes. After ischaemia, the raised [Na+]c causes a reversal of the sarcolemmal Na+/Ca2+ exchanger, leading to a raised [Ca2+]c and hence to the problems of Ca2+ overload discussed above [188–190]. This view is supported by the observation that decreased levels of Ca2+ in the perfusate ameliorate the effects of high [Na+]c [191]. However, too rapid a recovery of internal pH also seems to be damaging. Reperfusion damage can be reduced by slowing down the recovery of internal pH (i.e. keeping pHi low), by inhibiting the Na+/H+ exchanger and the Na+/HCO3− symport, possibly because this low pH inhibits the Ca2+ transients [4].
MPT (mitochondrial permeability transition)
When mitochondria are exposed to pathologically high levels of [Ca2+]m and various synergistic effectors they abruptly develop permeability to a range of small molecules (<1500 Da). This is known as the MPT. This leads to uncoupling, swelling, and loss of nucleotides (especially NAD+ and ADP) and other small molecules from the mitochondrial matrix [192]. ROS and non-esterified fatty acids are also known to promote the MPT. If the transition is prolonged, it is presumably pathological, as mitochondria so permeabilized will be unable to retain substrates or Δψ, ATP and NAD+ will be hydrolysed, and the cell will die by necrosis.
The MPT pore seems to be formed from an assembly of otherwise innocuous mitochondrial proteins, including peptidyl prolyl isomerase (cyclophilin D) from the mitochondrial matrix, the adenine nucleotide carrier from the inner membrane and the VDAC (voltage-dependent anion channel) of the outer membrane [193]. However, it is not known how these components assemble to make the pore, nor whether other proteins are involved. Recent studies using microarray analysis have suggested that the pro-apoptotic BNIP3 (Bcl2/adenovirus E1B 19 kDa protein interacting protein) might contribute to the pore [194]. However, none of the known components would be expected to confer Ca2+ sensitivity on the system. One possibility for modulating the pore in a Ca2+-dependent manner is PKC. Baines et al. [195] have shown that PKCϵ might phosphorylate VDAC in the pore complex. Since the conditions of high [Ca2+]m and raised levels of ROS and non-esterified fatty acids, which promote the MPT, are also conditions that promote cell death on ischaemia/reperfusion, it seems likely that this transition is the cause of cell death under these conditions.
This view is supported by the ability of cyclosporin, which specifically prevents the MPT, to protect against cell death in ischaemia/reperfusion [196–198]. In perfused heart, using [3H]deoxyglucose as a marker for MPT in mitochondria, Halestrap et al. [199] have shown that the mitochondrial pore remains largely closed during ischaemia, but opens during the reperfusion period, which is when most cell death occurs. While it seems likely that much cell death following ischaemia/reperfusion is due to the relatively disordered process of necrosis, apoptosis may also be important [200,201], and a precise estimate of the relative importance of each may well depend on the marker used for apoptosis [202]. Borutaite et al. [10,203] have shown that typical apoptotic changes (cytochrome c release, caspase activation, DNA strand breaks) begin during ischaemia in heart, and intensify during reperfusion. Again, these changes have been pharmacologically linked to the MPT via cyclosporin inhibition [204], although it is still uncertain how this transition may be linked to the early event of cytochrome c release from the intermembrane space. In an attractive model, Halestrap et al. [199] suggest that a transient opening of the MPT pore (e.g in the areas surrounding an infarct) might lead to apoptosis (which requires ATP) whereas a prolonged opening would lead to necrosis (which occurs in the absence of ATP).
METABOLIC AND PHARMACOLOGICAL INTERVENTIONS IN ISCHAEMIA AND REPERFUSION
The effects of ischaemia and reperfusion on the cardiac cell are profound. In the cytoplasm, there is a decrease in energy charge, an increase in the NADH/NAD+ ratio and a general loss of nucleotides. The cytoplasmic pH falls, and [Na+]c and [Ca2+]c both rise, constituting serious ionic imbalances. There is an increased production of ROS and of long-chain non-esterified fatty acids, and the NADPH/glutathione system becomes more oxidized, leading to protein damage. All of these changes have an impact on mitochondrial function leading to inhibition and uncoupling of electron flow, activation of the mitochondrial permeability transition, and loss of intermembrane cytochrome c. We have argued above that the mitochondrion is a major participant in these effects, and also that it might be the final arbiter of cell death in the apoptotic or necrotic changes that occur in myocardial infarct.
Whatever the precise sequence of events leading to cell death, it is certainly the case that treatments that oppose the above changes can be usefully employed to correct against ischaemic/reperfusion injury. Actions aimed to increase the energy charge, to render the relatively high negative redox potential less negative, to increase pH, and to restore the physiological concentration of ions in cell compartments are helpful to promote cardiac cell survival and recovery. Similarly, agents which minimize the cytotoxic effects of harmful agents such as ROS and long-chain fatty acids may be protective. In many systems, studying the effects of such agents (e.g. specific inhibitors) has helped us to understand, as well as to treat the defect, although such studies in heart have on balance arguably been confusing rather than aiding an understanding of the primary cause of ischaemia/reperfusion injury. Various treatments that have been applied are summarized in Table 1.
Table 1. Pharmacological and biochemical targets for cardioprotection involving mitochondrial activities.
CPT-1, carnitine palmitoyl transferase-1; DEVD-CHO, acetyl-Asp-Glu-Val-Asp-CHO; PDH, pyruvate dehydrogenase.
| Treatment | Effect(s) | Comment(s) | Reference(s) |
|---|---|---|---|
| General Treatments | |||
| Fasting and diet | Complex | Lowers glycogen stores | [205–207] |
| Brief incubation at low O2 | Complex | Ischaemic preconditioning | [208] |
| Glucose/insulin | Promote glycolysis | May induce protein phosphorylation | [209–211] |
| Pyruvate | Metabolic substrate, inhibits fatty acid oxidation | Also protects against ROS | [212,213] |
| Inhibitors of fatty acid metabolism | |||
| Trimetazidine, ranolazine | Inhibit fatty acid dehydrogenases | [214] | |
| Oxfenicine and other CPT-I inhibitors | Inhibit fatty acid entry into mitochondria | [206,215] | |
| Malonate | Inhibits fatty acid oxidation | [216,217] | |
| Dichloroacetate | Inhibits PDH kinase | Promotes glucose utilization | [218] |
| Carnitine and its derivatives | Lowers acetyl-CoA/free CoA, increasing free CoA | Promotes glycolysis by decreasing inhibition of PDH | [219,220] |
| Niacin | Reduces plasma non-esterified fatty acids | May help NAD recovery | [29,221] |
| Inhibitors of mitochondrial respiration | |||
| Amytal, rotenone | Inhibits electron flow, complex I | Decreases ROS production | [30,222] |
| Myxothiazole | Inhibits electron flow, complex III | Antimycin and CN− not protective | [223] |
| Uncouplers (dinitrophenol) | Decrease mitochondrial membrane potential | Time/dose dependence, to avoid energy wastage | [223] |
| Oligomycin | Inhibits F1Fo-ATPase | Time/dose dependence, to avoid ATP depletion | [224] |
| Inhibitors of ion movement | |||
| Ruthenium Red | Blocks mitochondrial Ca2+ import | Can also block the sarcoplasmic reticulum Ca2+-channel | [225,226] |
| Nisoldipine; gallopamil | Block Ca2+ entry into cytoplasm | [227–229] | |
| Tetrodotoxin | Blocks Na+ entry into cytoplasm | Damages mitochondria during ischaemia | [63] |
| Amiloride | Blocks cytoplasmic Na+/H+ exchanger | Prevents Ca2+ overload, therefore protects against reoxygenation-induced hypercontracture | [150,230,231] |
| Diltiazem; SEA0400 (5-ethoxyaniline derivative) | Inhibits mitochondrial Na+/Ca2+ exchange | [232–234] | |
| Diazoxide; nicorandil | K+-channel opener | Reversed by 5-OH decanoate, decreases ROS and plays roles in other undefined mechanisms | [59,66] |
| Inhibitors of cell damage mechanisms | |||
| Cyclosporin | Prevents MPT opening | May also inhibit Ca2+/CAM-dependent protein phosphatase (calcineurin) | [197,235] |
| Propofol | Traps ROS | ROS promote MPT | [199,236] |
| DEVD-CHO | Caspase 3 inhibitor (prevents downstream steps in apoptosis) | Only partially effective | [203] |
| Dipyridamole; carvedilol; natural compounds such as vitamin E and resveratrol; N-acetylcysteine | Antioxidants | Restores GSSG/GSH balance | [237–240] |
| Chloramphenicol | Inhibits cytochrome P450 mono-oxygenases | Also inhibits mitochondrial protein synthesis | [241] |
| Effectors of signalling pathways | |||
| Sevoflurane and other anaesthetics | NADH increase on ischaemia is reduced, and total [NAD]+[NADH] is preserved | Uncontrolled effects on membrane permeability to ions | [242] |
| Bromoenol-lactone | Inhibits mitochondrial Ca2+-independent phospholipases | [243] | |
| Soluble factors released from preconditioned heart | Includes adenosine, catecholamines, NO, prostanoids, endorphins | See review | [244] |
| Adenosine | Many effects, including modulation of mK+ATP channel and protein kinase C | Other additional protective mechanisms include phosphorylation to 5′-AMP during reperfusion | [245,246] |
Metabolic interventions
Under normal circumstances, the mammalian heart relies largely on mitochondrial fatty acid oxidation for energy (for a review, see [14]). However, it exhibits a dynamic plasticity related to energy substrate preferences and can switch seamlessly to glycolytic production of ATP under hypoxic conditions [1,247]. Since mitochondrial function is impaired at the start of reperfusion, metabolic interventions that promote glucose catabolism over fatty acid oxidation should be beneficial.
Clinical and experimental evidence clearly indicate the beneficial effect of glucose–insulin–potassium administration in reducing ischaemic damage [248]. Glucose can generate ATP glycolytically and will promote the production of malonyl-CoA, which in turn inhibits carnitine palmitoyltransferase-1 and hence fatty acid oxidation in mitochondria [217]. Correspondingly, increased myocardial glycogen will also protect against ischaemia/reperfusion damage [249], and species such as the harp seal, with myocardial glycogen levels at 10 times that in the rat, are resistant to ischaemic insults [250].
The seemingly paradoxical observation that fasting is protective against ischaemic/reperfusion damage [207,251] can be explained by the fact that fasting, in fact, increases heart glycogen levels [252]. Insulin itself may also have a direct protective effect, since it is known to protect cardiac cells from apoptosis [253]. Other important metabolic approaches to the treatment of ischaemic heart disease are reviewed by Wolff et al. [254] and by Stanley [13]. An interesting study by Weinberg et al. [255] indicates that in kidney, at least, deleterious effects of hypoxia/reperfusion can be alleviated by perfusion with α-oxoglutarate and aspartate (generating ATP via anaerobic operation of the tricarboxylic acid cycle), and the authors suggest that this might be applicable to heart. Along these lines, oral supplementation with mixed essential amino acids appears to protect rat heart exposed to ischaemia and reperfusion [256]. It is possible that the preservation of mitochondrial production of high-energy phosphates plays a role in the attenuation of ischaemia and reperfusion injury.
A particularly intriguing metabolic intervention, which clearly protects the heart against ischaemia/reperfusion injury, is a brief period of ischaemia itself. This is the phenomenon of preconditioning, discussed above. Although a variety of metabolic changes occur during the preconditioning period, they mirror the changes occurring during a damaging bout of ischaemia, and it is difficult to identify factors generated during a short period of ischaemia that would protect against a longer one. Acute factors, such as K+ATP channel opening and F1 inhibition, are discussed above, but there are, in addition, some long-term effects involving protein induction and possible humoral effects (as discussed below).
Pharmacological interventions
A range of inhibitors of mitochondrial activities have been reported to prevent ischaemia/reperfusion damage. Electron-transfer inhibitors, acting in the first stages of the respiratory chain are believed to act by inhibiting the production of ROS (which are largely generated at complexes I and III). The action of ATP synthesis inhibitors is, however, complex. Uncouplers such as dinitrophenol (and endogenous UCPs) are protective, presumably by decreasing Δψ and decreasing the activity of the mitochondrial Ca2+ uniport, while oligomycin (which blocks F1Fo activity) presumably decreases wasteful ATP hydrolysis (as will the endogenous regulator protein, IF1). However, these two agents will have complementary deleterious effects too: uncouplers will increase ATP hydrolysis, while F1Fo inhibitors will increase Δψ and, presumably increase mitochondrial Ca2+ uptake. The observed outcome will depend on the relative importance of these various effects in the system under study.
The next group of protective agents listed in Table 1 are those which prevent ion movements, and in particular inhibit the abnormally high accumulation of Na+ by the cytoplasm, and of Ca2+ by the mitochondrion, both of which seem to lead to irreversible damage. Also included are those which open the mK+ATP channels, a process which is implicated in protection via preconditioning. Like all inhibitors, however, their action will not be totally specific: Ruthenium Red and diazoxide, for example, inhibit sarcoplasmic reticulum ion channels, as well as mitochondrial channels for these respective ions, and diazoxide also inhibits electron transfer. As a result, the fact that a particular inhibitor prevents ischaemic/reperfusion damage cannot be taken as unequivocal proof for the participation of a particular ion channel in causing cell death.
There are many ways for a cell to die, and attempts to target known pathways in cell death are discussed next. The ordered apoptotic pathway (involving cytochrome c release from the intermembrane space of mitochondria and the activation of caspases, but requiring ATP) may contribute to ischaemia/reperfusion damage [10], and inhibitors of this pathway are partially protective. However, the importance of the contribution of the apoptotic pathway is unclear. Many researchers have suggested an important role for prolonged opening of the mitochondrial transition pore (reviewed in [9,199]), leading to depolarization of the mitochondria and massive ATP depletion, and indeed cyclosporin, the classical inhibitor of this transition, is protective. However, as transient opening of this pore may be a step in the apoptotic pathway, the interpretation of the cyclosporin effect is not unambiguous. Finally, there are direct effects of high [Ca2+] and ROS levels on other cell processes: both initiate lipid damage (activation of phospholipases and lipid peroxidation respectively) and protein damage (activation of proteases and oxidation of SH groups repectively), which might lead to irreversible loss of other essential cellular functions. Antioxidants in general have been shown to prevent oxidative damage to lipids, proteins and DNA following cardiac ischaemia and reperfusion [237–240], and these have been reviewed in [240a,240b].
Protective proteins
Using protein quantification and genetic manipulation techniques, several families of proteins have been shown to be protective against ischaemia/reperfusion damage. Unsurprisingly, these include HSPs, but also involved are the mitochondrial UCPs. Both groups are overexpressed in conditions of ischaemia/reperfusion [257–259] and can protect from cardiac injury. Endogenous mitochondrial HSP60 and HSP10 have been overexpressed experimentally and exert a protective effect on mitochondria exposed to simulated ischaemia [260]. This protective effect leads to increased recovery of ATP levels, and of the activities of complex III and IV [261]. Another protein of interest might be the HSP90-related protein, tumour-necrosis-factor-receptor-associated protein-1, which is expressed in mitochondria, and has a striking homology with (cytoplasmic) HSP90, although as yet its function is unknown [262].
Finally, it should be mentioned that the anti-apoptotic Bcl-2 family of proteins can reduce cell death, following ischaemia/reperfusion, by preventing reperfusion-induced apoptosis. They appear to inhibit cytochrome c release from the mitochondria and prevent activation of caspases 3 and 9 [263–265]. The importance of apoptosis in ischaemia/reperfusion damage is controversial as discussed above, and has been reviewed by Borutaite and Brown [10].
CONCLUSIONS AND PERSPECTIVES
In summary, while a number of processes have been shown to contribute to ischaemia/reperfusion damage in the heart, the relative importance of these processes is not yet clear. Further experiments with animal models, in which the variables (duration of ischaemia, oxygen tension, opening of ion channels) are more closely controlled, will be necessary to establish the primary or most damaging factor. Conclusive results on mitochondrial ischaemia/reperfusion injury, applicable across several different models of the state, were rare for several reasons. (i) Results on respiration rate, particularly under state IV conditions, were inconsistent. (ii) Direct comparison of studies was difficult due to the various methods used to perform myocardial perfusion, and to isolate the mitochondria. Other sources of variability include the use of different model species, and different tissue preparations (in vivo perfusion, isolated organ studies, isolated cell preparations). (iii) There is insufficient data on time dependence to allow correlations between the activity of ion channels, or the MPT pore and reperfusion injury, and protection. (iv) The role of NO was uncertain, as conclusions relied on the wide use of different concentrations of NOS inhibitors, and non-enzymatic NO production was not considered. Future advances in this field will depend on the identification of experimental factors that might influence respiration, ATP synthesis and cell death, and the standardization of conditions so as to remove these potentially confounding factors. Nonetheless, the work summarized above shows that there is substantial evidence to implicate impaired mitochondrial function in pathological changes associated with ischaemia and reperfusion injury in the heart. Hence manipulating mitochondrial function might prove to be useful in minimizing, or reversing, these changes.
Some of the changes appear to be due to global changes in energy charge, or redox state, and thus may be susceptible to general treatments with, for example, creatine, or coenzyme Q. However, a more fruitful approach might be to identify the specific systems that are implicated in causing, or contributing to, these changes, and which may therefore be appropriate for investigation as specific therapeutic targets. The work outlined above suggests a range of such potential targets, including the F1Fo–ATPase complex (with or without its inhibitory protein), free radical generators (of both the respiratory chain and the cytochrome P450 systems), the MPT pore and mitochondrial ion channels, particularly mK+ATP.
Further work appears necessary to identify the relative importance of these various systems in pathogenesis, and indeed any cause/effect relationships between them. A variety of possible approaches is summarized below. (i) Significant advances in our understanding of metabolic and mitochondrial functional changes in ischaemia and reperfusion have come from in vitro studies. Clearly it would be advantageous to obtain more precise information in vivo from hearts. This could be accomplished with the development of highly resolving magnetic resonance spectroscopy and imaging. Work in vivo with probes should also include monitoring the time course of changes in mitochondrial membrane potential, ion fluxes and metabolite concentrations. (ii) The rapidly emerging field of proteomics will provide powerful tools to identify mitochondrial proteins differentially expressed in normal and disease states. This will allow the identification of proteins involved directly in ischaemia and reperfusion associated with, or caused by, mitochondrial dysfunction. Future efforts will focus on linking genomic array information to actual protein levels in mitochondria in relation to their functions. (iii) Developments are needed in characterizing protein–protein interactions, which profoundly influence metabolic control. The interaction surface between the inhibitor protein and the mitochondrial ATPase, for example, has now been identified [266]. Knowledge of the specific amino acid side chains that are involved in such interactions should allow pharmacological, or even genetic, interventions to increase the protection of cardiomyocytes from ischaemia and reperfusion.
Therapy is, in fact, mostly based on the administration of various compounds, alone or in combination. Controlled clinical investigations, on human subjects, will be needed to judge the efficacy and safety of drug regimens, but this should be informed by the basic scientific investigations outlined above. We suggest that clinicians and researchers in basic science set up common projects to design studies on patients, in parallel with animal models, paying particular attention to the first several hours after an ischaemic insult.
We hope that this review contributes to an understanding of the central role of mitochondria in energy metabolism and ion homoeostasis in heart ischaemia and reperfusion, and, paraphrasing the words of Lopaschuk and Opie [267], it might “…help to re-instate metabolism and bioenergetics as proper supports of cardiology”.
Acknowledgments
This study was supported by a grant from Scuola Superiore S. Anna, Pisa, Italy (MRIS01GS). We also acknowledge the skilful technical help of Dr Gianluca Sgarbi in drawing the schemes.
References
- 1.Das A. M., Harris D. A. Regulation of the mitochondrial ATP synthase in intact rat cardiomyocytes. Biochem. J. 1990;266:355–361. doi: 10.1042/bj2660355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dennis S. C., Gevers W., Opie L. H. Protons in ischemia: where do they come from; where do they go? J. Mol. Cell. Cardiol. 1991;23:1077–1086. doi: 10.1016/0022-2828(91)91642-5. [DOI] [PubMed] [Google Scholar]
- 3.Schlüter K. D., Jakob G., Ruiz-Meana M., García-Dorado D., Piper H. M. Protection of reoxygenated cardiomyocytes against osmotic fragility by NO donors. Am. J. Physiol. 1996;271:H428–H434. doi: 10.1152/ajpheart.1996.271.2.H428. [DOI] [PubMed] [Google Scholar]
- 4.Piper H. M., Abdallah Y., Schaefer C. The first minutes of reperfusion: a window of opportunity for cardioprotection. Cardiovasc. Res. 2004;61:365–371. doi: 10.1016/j.cardiores.2003.12.012. [DOI] [PubMed] [Google Scholar]
- 5.Ferrari R. The role of mitochondria in ischemic heart disease. J. Cardiovasc. Pharmacol. 1996;28:S1: 1–10. doi: 10.1097/00005344-199600003-00002. [DOI] [PubMed] [Google Scholar]
- 6.Lesnefsky E. J., Moghaddas S., Tandler B., Kerner J., Hoppel C. L. Mitochondrial dysfunction in cardiac disease: ischemia-reperfusion, aging, and heart failure. J. Mol. Cell. Cardiol. 2001;33:1065–1089. doi: 10.1006/jmcc.2001.1378. [DOI] [PubMed] [Google Scholar]
- 7.Pepe S. Mitochondrial function in ischaemia and reperfusion of the ageing heart. Clin. Exp. Pharmacol. Physiol. 2000;27:745–750. doi: 10.1046/j.1440-1681.2000.03326.x. [DOI] [PubMed] [Google Scholar]
- 8.Piper H. M., Noll T., Siegmund B. Mitochondrial function in the oxygen depleted and reoxygenated myocardial cell. Cardiovasc. Res. 1994;28:1–15. doi: 10.1093/cvr/28.1.1. [DOI] [PubMed] [Google Scholar]
- 9.Di Lisa F., Canton M., Menabò R., Bodoni G., Bernardi P. Mitochondria and reperfusion injury. Basic Res. Cardiol. 2003;98:235–241. doi: 10.1007/s00395-003-0415-x. [DOI] [PubMed] [Google Scholar]
- 10.Borutaite V., Brown G. C. Mitochondria in apoptosis of ischaemic heart. FEBS Lett. 2003;541:1–5. doi: 10.1016/s0014-5793(03)00278-3. [DOI] [PubMed] [Google Scholar]
- 11.Flaherty J. T., Weisfeldt M. L. Reperfusion injury. Free Radical Biol. Med. 1988;5:409–419. doi: 10.1016/0891-5849(88)90115-3. [DOI] [PubMed] [Google Scholar]
- 12.Hachida M., Lu H., Ohkado A., Hoshi H., Gu H., Nakanishi T., Koyanagi H. Effectiveness of ischemic preconditioning on long-term myocardial preservation. Transplantation. 1998;65:1021–1024. doi: 10.1097/00007890-199804270-00002. [DOI] [PubMed] [Google Scholar]
- 13.Stanley W. C. Coronary energetics during ischemia and the rationale for metabolic interventions Coron. Artery Dis. 2001;12:S1: 3–7. [PubMed] [Google Scholar]
- 14.Sun K. T., Yeatman L. A., Buxton D. B., Chen K., Johnson J. A., Huang S. C., Kofoed K. F., Weismueller S., Czemin J., Phelps M. E., Schelbert H. R. Simultaneous measurement of myocardial oxygen consumption and blood flow using [1-C11]acetate. J. Nucl. Med. 1988;39:272–280. [PubMed] [Google Scholar]
- 15.Fridovich I. Superoxide radical and superoxide dismutase. Annu. Rev. Biochem. 1995;64:97–112. doi: 10.1146/annurev.bi.64.070195.000525. [DOI] [PubMed] [Google Scholar]
- 16.Dobson G. P., Himmelreich U. Heart design: free ADP scales with absolute mitochondrial and myofibrillar volumes from mouse to human. Biochim. Biophys. Acta. 2002;1553:261–267. doi: 10.1016/s0005-2728(01)00247-x. [DOI] [PubMed] [Google Scholar]
- 17.Palmer J. V., Tandler B., Hoppel C. L. Biochemical properties of subsarcolemmal and interfibrillar mitochondria isolated from rat cardiac muscle. J. Biol. Chem. 1977;252:8731–8739. [PubMed] [Google Scholar]
- 18.Opie L. H. NY: Raven Press; 1991. The Heart: Physiology and Metabolism. [Google Scholar]
- 19.Reimer K. A., Jennings R. B. Myocardial ischemia, hypoxia and infarction. In: Fozzard H. A., Haber E., Jennings R. B., Katz A. M., Morgan H. E., editors. The Heart and Circulatory System. NY: Raven Press; 1986. pp. 1133–1201. [Google Scholar]
- 20.Maloney P. C. Energy coupling to ATP synthesis by the proton-translocating ATPase. J. Membr. Biol. 1982;67:1–12. doi: 10.1007/BF01868643. [DOI] [PubMed] [Google Scholar]
- 21.Saks V. A., Kongas O., Vendelin M., Kay L. Role of the creatine/phosphocreatine system in the regulation of mitochondrial respiration. Acta Physiol. Scand. 2000;168:635–641. doi: 10.1046/j.1365-201x.2000.00715.x. [DOI] [PubMed] [Google Scholar]
- 22.Varadarajan S. G., An J., Novalija E., Smart S. C., Stowe D. F. Changes in [Na+](i), compartmental [Ca2+], and NADH with dysfunction after global isch emia in intact hearts. Am. J. Physiol. Heart Circ. Physiol. 2001;280:H280–H293. doi: 10.1152/ajpheart.2001.280.1.H280. [DOI] [PubMed] [Google Scholar]
- 23.Miyata H., Silverman H. S., Sollott S. J., Lakatta E. G., Stern M. D., Hansford R. G. Measurement of mitochondrial free Ca2+ concentration in living single rat cardiac myocytes. Am. J. Physiol. 1991;261:H1123–H1134. doi: 10.1152/ajpheart.1991.261.4.H1123. [DOI] [PubMed] [Google Scholar]
- 24.Denton R. M., McCormack J. G. Ca2+ transport by mammalian mitochondria and its role in hormone action. Am. J. Physiol. 1985;249:E543–E554. doi: 10.1152/ajpendo.1985.249.6.E543. [DOI] [PubMed] [Google Scholar]
- 24a.Rizzuto R., Bernardi P., Pozzan T. Mitochondria as all-round players of the calcium game. J. Physiol. 2000;529:37–47. doi: 10.1111/j.1469-7793.2000.00037.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Neely J. R., Rovetto M. J., Whitmer J. T., Morgan H. E. Effects of ischemia on function and metabolism of the isolated working rat heart. Am. J. Physiol. 1973;225:651–658. doi: 10.1152/ajplegacy.1973.225.3.651. [DOI] [PubMed] [Google Scholar]
- 26.Lopaschuk G. D., Belke D. D., Gamble J., Itoi T., Schonekness B. O. Regulation of fatty acid oxidation in the mammalian heart and disease. Biochim. Biophys. Acta. 1994;1213:263–276. doi: 10.1016/0005-2760(94)00082-4. [DOI] [PubMed] [Google Scholar]
- 27.Vuorinen K., Ylitalo K., Peuhkurinen K., Raatikainen P., Ala Rami A., Hassinen I. E. Mechanisms of ischemic preconditioning in rat myocardium. Roles of adenosine, cellular energy state, and mitochondrial F1Fo-ATPase. Circulation. 1995;91:2810–2818. doi: 10.1161/01.cir.91.11.2810. [DOI] [PubMed] [Google Scholar]
- 28.Smith D. R., Stone D., Darley-Usmar V. M. Stimulation of mitochondrial oxygen consumption in isolated cardiomyocytes after hypoxia-reoxygenation. Free Radical Res. 1996;24:159–166. doi: 10.3109/10715769609088013. [DOI] [PubMed] [Google Scholar]
- 29.Trueblood N. A., Ramasamy R., Wang L. F., Scaefer S. Niacin protects the isolated heart from ischemia-reperfusion injury. Am. J. Physiol. Heart Circ. Physiol. 2000;279:H764–H771. doi: 10.1152/ajpheart.2000.279.2.H764. [DOI] [PubMed] [Google Scholar]
- 30.Ambrosio G., Zweier J. L., Duilio C., Kuppusamy P., Sanoro G., Elia P. P., Tritto J., Cirillo P., Condorelli M., Chiarello M. Evidence that mitochondrial respiration is a source of potentially toxic oxygen free radicals in intact rabbit hearts subjected to ischemia and reflow. J. Biol. Chem. 1993;268:18532–18541. [PubMed] [Google Scholar]
- 31.Schaper J., Schaper W. Reperfusion of ischemic myocardium: ultrastructural and histochemical aspects. J. Am. Coll. Cardiol. 1983;1:1037–1046. doi: 10.1016/s0735-1097(83)80106-5. [DOI] [PubMed] [Google Scholar]
- 32.Di Lisa F., Menabo R., Canton M., Petronilli V. The role of mitochondria in the salvage and the injury of the ischaemic myocardium. Biochim. Biophys. Acta. 1998;1366:69–78. doi: 10.1016/s0005-2728(98)00121-2. [DOI] [PubMed] [Google Scholar]
- 33.Nunez R., Calva E., Marsch M., Briones E., Lopez-Soriano F. NAD glycohydrolase activity in hearts with acute experimental infarction. Am. J. Physiol. 1976;231:1173–1177. doi: 10.1152/ajplegacy.1976.231.4.1173. [DOI] [PubMed] [Google Scholar]
- 34.Ziegler D. M. Role of reversible oxidation-reduction enzyme thiols-disulfides in metabolic regulation. Annu. Rev. Biochem. 1985;54:305–329. doi: 10.1146/annurev.bi.54.070185.001513. [DOI] [PubMed] [Google Scholar]
- 35.Ceconi C., Bernocchi P., Borsano A., Cagnoni A., Pepi P., Curello S., Ferrari R. New insights on myocardial pyridine nucleotides and thiol redox state in ischemia and reperfusion damage. Cardiovasc. Res. 2000;47:586–594. doi: 10.1016/s0008-6363(00)00104-8. [DOI] [PubMed] [Google Scholar]
- 36.Hwang Y. C., Sato S., Tsai J. Y., Yan S., Bakr S., Zhang H., Oates P. J., Ramasamy R. Aldose reductase activation is a key component of myocardial response to ischaemia. FASEB J. 2002;16:243–245. doi: 10.1096/fj.01-0368fje. [DOI] [PubMed] [Google Scholar]
- 37.Jennings R. B., Reimer K. A., Steenbergen C. Effect of inhibition of the mitochondrial ATPase on net myocardial ATP in total ischemia. J. Mol. Cell. Cardiol. 1991;23:1383–1395. doi: 10.1016/0022-2828(91)90185-o. [DOI] [PubMed] [Google Scholar]
- 38.Kobara M., Tatsumi T., Matoba S., Yamahara Y., Nakagawa C., Ohta B., Matsumoto T., Inoue D., Asayama J., Nakagawa M. Effect of ischemic preconditioning on mitochondrial oxidative phosphorylation and high energy phosphates in rat hearts. J. Mol. Cell. Cardiol. 1996;28:417–428. doi: 10.1006/jmcc.1996.0038. [DOI] [PubMed] [Google Scholar]
- 39.Casey T. M., Pakay J. L., Guppy M., Arthur P. G. Hypoxia causes downregulation of protein and RNA synthesis in noncontracting mammalian cardiomyocytes. Circ. Res. 2002;90:777–783. doi: 10.1161/01.res.0000015592.95986.03. [DOI] [PubMed] [Google Scholar]
- 40.Damy T., Ratajczak P., Robidel E., Bendall J. K., Oliviero P., Boczkowski J., Ebrahimian T., Marotte F., Samuel J. L., Heymes C. Up-regulation of cardiac nitric oxide synthase 1-derived nitric oxide after myocardial infarction in senescent rats. FASEB J. 2003;13:1934–1936. doi: 10.1096/fj.02-1208fje. [DOI] [PubMed] [Google Scholar]
- 41.Ping P., Song C., Zhang J., Guo Y., Cao X., Li R. C., Wu W., Vondriska T. M., Pass J. M., Tang X. L., Pierce W. M., Bolli R. Formation of protein kinase Cϵ–Lck signaling modules confers cardioprotection. J. Clin. Invest. 2002;109:499–507. doi: 10.1172/JCI13200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Levy F. H., Kelly D. P. Regulation of ATP synthase subunit e gene expression by hypoxia: cell differentiation stage-specific control. Am. J. Physiol. 1997;272:457–465. doi: 10.1152/ajpcell.1997.272.2.C457. [DOI] [PubMed] [Google Scholar]
- 43.Ladilov Y. V., Siegmund B., Piper H. M. Protection of reoxygenated cardiomyocytes against hypercontracture by inhibition of Na+/H+ exchange. Am. J. Physiol. 1995;268:H1531–H1539. doi: 10.1152/ajpheart.1995.268.4.H1531. [DOI] [PubMed] [Google Scholar]
- 44.Kajstura J., Cheng W., Reiss K., Clark W. A., Sonnenblick E. H., Krajewski S., Reed J. C., Olivetti G., Anversa P. Apoptotic and necrotic myocyte cell deaths are independent contributing variables of infarct size in rats. Lab. Invest. 1996;74:86–107. [PubMed] [Google Scholar]
- 45.McCord J. M. Free radicals and myocardial ischemia: overview and outlook. Free Radical Biol. Med. 1988;4:9–14. doi: 10.1016/0891-5849(88)90005-6. [DOI] [PubMed] [Google Scholar]
- 46.Zuurbier C. J., Erebeek O., Goedhart P. T., Struys E. A., Verhoeven N. M., Jakobs C., Ince C. Inhibition of the pentose phosphate pathway decreases ischemia-reperfusion-induced creatine kinase release in the heart. Cardiovasc. Res. 2004;62:145–153. doi: 10.1016/j.cardiores.2004.01.010. [DOI] [PubMed] [Google Scholar]
- 47.Yellon D. M., Baxter G. F., Garcia-Dorado D., Heusch G., Sumeray M. S. Ischemic preconditioning: present position and future directions. Cardiovasc. Res. 1998;37:21–33. doi: 10.1016/s0008-6363(97)00214-9. [DOI] [PubMed] [Google Scholar]
- 48.Takeo S., Nasa Y. Role of energy metabolism in the preconditioned heart –a possible contribution of mitochondria. Cardiovasc. Res. 1999;43:32–43. doi: 10.1016/s0008-6363(99)00079-6. [DOI] [PubMed] [Google Scholar]
- 49.Minners J., McLeod C. J., Sack M. N. Mitochondrial plasticity in classical ischemic preconditioning – moving beyond the mitochondrial KATP channel. Cardiovasc. Res. 2003;59:1–6. doi: 10.1016/s0008-6363(03)00337-7. [DOI] [PubMed] [Google Scholar]
- 50.Kaliulin I., Schwalb H., Wang P., Houminer E., Grinberg L., Katzeff H., Borman J. B., Powell S. R. Preconditioning improves postischemic mitochondrial function and diminishes oxidation of mitochondrial proteins. Free Radical Biol. Med. 2004;37:1–9. doi: 10.1016/j.freeradbiomed.2004.04.017. [DOI] [PubMed] [Google Scholar]
- 51.Jennings R. B., Herdson P. B., Sommer H. M. Structural and functional abnormalities in mitochondria isolated from ischemic dog myocardium. Lab. Invest. 1969;20:548–557. [PubMed] [Google Scholar]
- 52.Toleikis A., Dzja P., Praskevicius A., Jasaitis A. Mitochondrial functions in ischemic myocardium. I. Proton electrochemical gradient, inner membrane permeability, calcium transport and oxidative phosphorylation in isolated mitochondria. J. Mol. Cell. Cardiol. 1979;11:57–76. doi: 10.1016/0022-2828(79)90452-8. [DOI] [PubMed] [Google Scholar]
- 53.Di Lisa F., Menabò R., Barbato R., Siliprandi N. Contrasting effects of propionate and propionyl-L-carnitine on energy-linked processes in ischemic hearts. Am. J. Physiol. 1994;267:H455–H461. doi: 10.1152/ajpheart.1994.267.2.H455. [DOI] [PubMed] [Google Scholar]
- 54.Cairns C. B., Ferroggiaro A. A., Walther J. M., Harken A. H., Banerjee A. Postischemic administration of succinate reverses the impairment of oxidative phosphorylation after cardiac ischemia and reperfusion injury. Circulation. 1997;96:260–265. [PubMed] [Google Scholar]
- 55.Lucas D. T., Szweda L. I. Declines in mitochondrial respiration during cardiac reperfusion: age-dependent inactivation of alpha-ketoglutarate dehydrogenase. Proc. Natl. Acad. Sci. U.S.A. 1999;96:6689–6693. doi: 10.1073/pnas.96.12.6689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hardy L., Clark J. B., Darley-Usmar V. M., Smith D. R., Stone D. Reoxygenation-dependent decrease in mitochondrial NADH:CoQ reductase (Complex I) activity in the hypoxic/reoxygenated rat heart. Biochem. J. 1991;274:133–137. doi: 10.1042/bj2740133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bosetti F., Baracca A., Lenaz G., Solaini G. Increased state 4 mitochondrial respiration and swelling in early-post ischemic reperfusion of rat heart. FEBS Lett. 2004;563:161–164. doi: 10.1016/S0014-5793(04)00294-7. [DOI] [PubMed] [Google Scholar]
- 58.Sako E. Y., Kingsley-Hickman P. B., From A. H., Foker J. E., Ugurbil K. ATP synthesis kinetics and mitochondrial function in the postischemic myocardium as studied by 31P NMR. J. Biol. Chem. 1988;263:10600–10607. [PubMed] [Google Scholar]
- 59.Lim K. H., Javadov S. A., Das M., Clarke S. J., Suleiman M. S., Halestrap A. P. The effects of ischemic preconditioning, diazoxide and 5-hydroxydecanoate on rat heart mitochondrial volume and respiration. Am. J. Physiol. Heart Circ. Physiol. 2002;545:961–974. doi: 10.1113/jphysiol.2002.031484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Minners J., Lacerda L., McCarthy J., Meiring J. J., Yellon D. M., Sack M. N. Ischemic and pharmacological preconditioning in Girardi cells and C2C12 myotubes induce mitochondrial uncoupling. Circ. Res. 2001;89:787–792. doi: 10.1161/hh2101.098372. [DOI] [PubMed] [Google Scholar]
- 61.Taniguchi M., Wilson C., Hunter C. A., Pehowich D. J., Clanachan A. S., Lopaschuk G. D. Dichloroacetate improves cardiac efficiency after ischemia independent of changes in mitochondrial proton leak. Am. J. Physiol. Heart Circ. Physiol. 2001;280:H762–H769. doi: 10.1152/ajpheart.2001.280.4.H1762. [DOI] [PubMed] [Google Scholar]
- 62.Borutaite V., Mildaziene V., Brown G. C., Brand M. D. Control and kinetic analysis of ischaemia-damaged heart mitochondria: which parts of the oxidative phosphorylation system are affected by ischaemia? Biochim. Biophys. Acta. 1995;1272:154–158. doi: 10.1016/0925-4439(95)00080-1. [DOI] [PubMed] [Google Scholar]
- 63.Iwai T., Tanonaka K., Inoue R., Kasahara S., Motegi K., Nagaya S., Takeo S. Sodium accumulation during ischemia induces mitochondrial damage in perfused rat hearts. Cardiovas. Res. 2002;55:141–149. doi: 10.1016/s0008-6363(02)00282-1. [DOI] [PubMed] [Google Scholar]
- 64.Palmer J. V., Tandler B., Hoppel C. L. Biochemical properties of subsarcolemmal and interfibrillar mitochondria isolated from rat cardiac muscle. J. Biol. Chem. 1977;252:8731–8739. [PubMed] [Google Scholar]
- 65.Vendelin M., Eimre M., Seppet E., Peet N., Andrienko T., Lemba M., Engelbrecht J., Seppet E. K., Saks V. A. Intracellular diffusion of adenosine phosphates is locally restricted in cardiac muscle. Mol. Cell. Biochem. 2004;256:229–241. doi: 10.1023/b:mcbi.0000009871.04141.64. [DOI] [PubMed] [Google Scholar]
- 66.Ozcan C., Bienengraeber M., Dzeja P. P., Terzic A. Potassium channel openers protect cardiac mitochondria by attenuating oxidant stress at reoxygenation. Am. J. Physiol. Heart Circ. Physiol. 2002;282:H531–H539. doi: 10.1152/ajpheart.00552.2001. [DOI] [PubMed] [Google Scholar]
- 67.Rouslin W. Mitochondrial complexes I, II, III, IV, and V in myocardial ischemia and autolysis. Am. J. Physiol. 1983;244:H743–H748. doi: 10.1152/ajpheart.1983.244.6.H743. [DOI] [PubMed] [Google Scholar]
- 68.Veitch K., Hombroeckx A., Caucheteux D., Pouleur H., Hue L. Global ischaemia induces a biphasic response of the mitochondrial respiratory chain. Anoxic pre-perfusion protects against ischaemic damage. Biochem. J. 1992;281:709–715. doi: 10.1042/bj2810709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Cairns C. B., Ferroggiaro A. A., Walther J. M., Harken A. H., Banerjee A. Postischaemic administration of succinate reverses the impairment of oxidative phosphorylation after cardiac ischaemia and reperfusion injury. Circulation. 1997;96(II):260–265. [PubMed] [Google Scholar]
- 70.Paradies G., Pedrosillo G., Pistolese M., Di Venosa N., Federici A., Ruggiero F. M. Decrease in mitochondrial complex I activity in ischaemic/reperfused rat heart: involvement of reactive oxygen species and cardiolipin. Circ. Res. 2004;94:53–59. doi: 10.1161/01.RES.0000109416.56608.64. [DOI] [PubMed] [Google Scholar]
- 71.Sadek H. A., Humphries K. M., Szweda P. A., Szweda L. I. Selective inactivation of redox-sensitive mitochondrial enzymes during cardiac reperfusion. Arch. Biochem. Biophys. 2002;406:222–228. doi: 10.1016/s0003-9861(02)00446-0. [DOI] [PubMed] [Google Scholar]
- 72.Geshi E., Konno N., Yanagishita T., Katagiri T. Impairment of mitochondrial respiratory activity in the early ischemic myocardium with special reference to electron transport system. Jpn. Circ. J. 1988;52:535–542. doi: 10.1253/jcj.52.535. [DOI] [PubMed] [Google Scholar]
- 73.Rouslin W. Effects of acidosis and ATP depletion on cardiac muscle electron transfer complex I. J. Mol. Cell. Cardiol. 1991;23:1127–1135. doi: 10.1016/0022-2828(91)90202-w. [DOI] [PubMed] [Google Scholar]
- 74.Maklashina E., Sher Y., Zhou H. Z., Gray M. O., Karliner J. S., Cecchini G. Effect of anoxia/reperfusion on the reversible active/de-active transition of NADH-ubiquinone oxidoreductase (complex I) in rat heart. Biochim. Biophys. Acta. 2002;1556:6–12. doi: 10.1016/s0005-2728(02)00280-3. [DOI] [PubMed] [Google Scholar]
- 75.Paradies G., Pedrosillo G., Pistolese M., Ruggiero F. M. Reactive oxygen species affect mitochondrial electron transport complex I activity through oxidative cardiolipin damage. Gene. 2002;286:135–141. doi: 10.1016/s0378-1119(01)00814-9. [DOI] [PubMed] [Google Scholar]
- 76.Abe K., Hayashi N., Terada H. Effect of endogenous nitric oxide on energy metabolism of rat heart mitochondria durino ischemia and reperfusion. Free Radical Biol. Med. 1999;26:779–787. doi: 10.1016/s0891-5849(98)00222-6. [DOI] [PubMed] [Google Scholar]
- 77.Riobò N. A., Clementi E., Melani M., Boveris A., Cadenas E., Moncada S., Poderoso J. J. Nitric oxide inhibits mitochondrial NADH:ubiquinone reductase activity through peroxynitrite formation. Biochem. J. 2001;359:139–145. doi: 10.1042/0264-6021:3590139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Jekabsone A., Ivanoviene L., Brown G. C., Borutaite V. Nitric oxide and calcium together inactivate mitochondrial complex I and induce cytochrome c release. J. Mol. Cell. Cardiol. 2003;35:803–809. doi: 10.1016/s0022-2828(03)00137-8. [DOI] [PubMed] [Google Scholar]
- 78a.Moreno-Sanchez R., Devars S., Lopez-Gomez F., Uribe A., Corona N. Distribution of the control of oxidative phosphorylation in mitochondria oxidising NAD-linked substrates. Biochim. Biophys. Acta. 1991;1060:284–292. doi: 10.1016/s0005-2728(05)80318-4. [DOI] [PubMed] [Google Scholar]
- 78b.Rossignol R., Letellier T., Malgat M., Rocher C., Mazat J. P. Tissue variation in the control of oxidative phosphorylation; implication for mitochondrial diseases. Biochem. J. 2000;347:45–53. [PMC free article] [PubMed] [Google Scholar]
- 79.Sako E. Y., Kingsley-Hickman P. B., From A. H., Foker J. E., Ugurbil K. ATP synthesis kinetics and mitochondrial function in the postischaemic myocardium as studied by 31P NMR. J. Biol. Chem. 1988;263:10600–10607. [PubMed] [Google Scholar]
- 80.Neubauer S., Hamman B. L., Perry S. B., Bittl J. A., Ingwall J. S. Velocity of the creatine kinase reaction decreases in postischaemic myocardium: a 31P-NMR magnetization transfer study of the isolated ferret heart. Circ. Res. 1988;63:1–15. doi: 10.1161/01.res.63.1.1. [DOI] [PubMed] [Google Scholar]
- 81.Piper H. M., Sezer O., Schleyer M., Schwartz P., Hutter J. F., Spieckermann P. G. Development of ischemia-induced damage in defined mitochondrial subpopulations. J. Mol. Cell. Cardiol. 1985;17:885–896. doi: 10.1016/s0022-2828(85)80102-4. [DOI] [PubMed] [Google Scholar]
- 82.Hoppel C. L., Moghaddas S., Lesnefsky E. J. Interfibrillar cardiac mitochondrial complex III defects in the aging rat heart. Biogerontology. 2002;3:41–44. doi: 10.1023/a:1015251212039. [DOI] [PubMed] [Google Scholar]
- 83.Petrosillo G., Ruggiero F. M., Di Venosa N., Paradies G. Decreased complex III activity in mitochondria isolated from rat heart subjected to ischemia and reperfusion: role of reactive oxygen species and cardiolipin. FASEB J. 2003;17:714–716. doi: 10.1096/fj.02-0729fje. [DOI] [PubMed] [Google Scholar]
- 84.Guidarelli A., Cantoni O. Pivotal role of superoxides generated in the mitochondrial respiratory chain in peroxynitrite-dependent activation of phospholipase A2. Biochem. J. 2002;366:307–14. doi: 10.1042/BJ20020284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Borutaite V., Morkuniene R., Budriunaite A., Krasauskaite D., Ryselis S., Toleikis A., Brown G. C. Kinetic analysis of changes in activity of heart mitochondrial oxidative phosphorylation system induced by ischemia. J. Mol. Cell. Cardiol. 1996;28:2195–2201. doi: 10.1006/jmcc.1996.0211. [DOI] [PubMed] [Google Scholar]
- 86.Lesnefsky E. J., Tandler B., Ye J., Slabe T. J., Turkaly J., Hoppel C. L. Myocardial ischemia decreases oxidative phosphorylation through cytochrome oxidase in subsarcolemmal mitochondria. Am. J. Physiol. 1997;273:H1544–H1554. doi: 10.1152/ajpheart.1997.273.3.H1544. [DOI] [PubMed] [Google Scholar]
- 87.Paradies G., Petrosillo G., Pistolese M., Di Venosa N., Serena D., Ruggiero F. M. Lipid peroxidation and alterations to oxidative metabolism in mitochondria isolated from rat heart subjected to ischemia and reperfusion. Free Radical Biol. Med. 1999;27:42–50. doi: 10.1016/s0891-5849(99)00032-5. [DOI] [PubMed] [Google Scholar]
- 88.Lesnefsky E. J., Chen Q., Slabe T. J., Stoll M. S., Minkler P. E., Hassan M. O., Tandler B., Hoppel C. L. Ischemia, rather than reperfusion, inhibits respiration through cytochrome oxidase in the isolated, perfused rabbit heart: role of cardiolipin. Am. J. Physiol. Heart Circ. Physiol. 2004;1:H258–H267. doi: 10.1152/ajpheart.00348.2003. [DOI] [PubMed] [Google Scholar]
- 89.Chen J., Henderson G. I., Freeman G. L. Role of 4-hydroxynonenal in modification of cytochrome c oxidase in ischemia/reperfused rat heart. J. Mol. Cell. Cardiol. 2001;33:1919–1927. doi: 10.1006/jmcc.2001.1454. [DOI] [PubMed] [Google Scholar]
- 90.Gnaiger E., Mendez G., Hand S. C. High phosphorylation efficiency and depression of uncoupled respiration in mitochondria under hypoxia. Proc. Natl. Acad. Sci. U.S.A. 2000;97:11080–11085. doi: 10.1073/pnas.97.20.11080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Gnaiger E., Kuznetsov A. V. Mitochondrial respiration at low levels of oxygen and cytochrome c. Biochem. Soc. Trans. 2002;30:252–258. doi: 10.1042/. [DOI] [PubMed] [Google Scholar]
- 92.Kunz W. S., Kudin A., Vielhaber S., Elger C. E., Attardi G., Villani G. Flux control of cytochrome c oxidase in human skeletal muscle. J. Biol. Chem. 2000;275:27741–27745. doi: 10.1074/jbc.M004833200. [DOI] [PubMed] [Google Scholar]
- 93.Turrens J. F. Superoxide production by the mitochondrial respiratory chain. Biosci. Rep. 1997;17:3–8. doi: 10.1023/a:1027374931887. [DOI] [PubMed] [Google Scholar]
- 94.Rao P. S., Mueller H. S. Lipid peroxidation and acute myocardial ischemia. Adv. Exp. Med. Biol. 1983;161:347–363. doi: 10.1007/978-1-4684-4472-8_19. [DOI] [PubMed] [Google Scholar]
- 95.Becker L. B., Vanden Hoek T. L., Shao Z. H., Li C. Q., Schumacker P. T. Generation of superoxide in cardiomyocytes during ischemia before reperfusion. Am. J. Physiol. 1999;277:H2240–H2246. doi: 10.1152/ajpheart.1999.277.6.H2240. [DOI] [PubMed] [Google Scholar]
- 96.Du G., Mouithys-Mickalad A., Sluse F. E. Generation of superoxide anion by mitochondria and impairment of their functions during anoxia and reoxygenation in vitro. Free Radical Biol. Med. 1998;25:1066–1074. doi: 10.1016/s0891-5849(98)00148-8. [DOI] [PubMed] [Google Scholar]
- 97.Wickens A. P. Ageing and the free radical theory. Respir. Physiol. 2001;128:379–391. doi: 10.1016/s0034-5687(01)00313-9. [DOI] [PubMed] [Google Scholar]
- 98.Vanden Hoek T. L., Becker L. B., Shao Z., Li C., Schumacker P. T. Reactive oxygen species released from mitochondria during brief hypoxia induce preconditioning in cardiomyocytes. J. Biol. Chem. 1998;273:18092–18098. doi: 10.1074/jbc.273.29.18092. [DOI] [PubMed] [Google Scholar]
- 99.Kulisz A., Chen N., Chandel N. S., Shao Z., Schumacker P. T. Mitochondrial ROS initiate phosphorylation of p38 MAP kinase during hypoxia in cardiomyocytes. Am. J. Physiol. Lung Cell. Mol. Physiol. 2002;282:L1324–L1329. doi: 10.1152/ajplung.00326.2001. [DOI] [PubMed] [Google Scholar]
- 100.Finkel T. Oxygen radicals and signalling. Curr. Opin. Cell Biol. 1998;10:248–253. doi: 10.1016/s0955-0674(98)80147-6. [DOI] [PubMed] [Google Scholar]
- 101.Kang P. M., Izumo S. Apoptosis and heart failure: A critical review of the literature. Circ. Res. 2000;86:1107–1113. doi: 10.1161/01.res.86.11.1107. [DOI] [PubMed] [Google Scholar]
- 102.Levraut J., Iwase H., Shao Z. H., Vanden Hoek T. L., Schumacker P. T. Cell death during ischemia: relationship to mitochondrial depolarization and ROS generation. Am. J. Physiol. Heart Circ. Physiol. 2003;284:H549–H558. doi: 10.1152/ajpheart.00708.2002. [DOI] [PubMed] [Google Scholar]
- 103.Kinnula V. L., Crapo J. D. Superoxide dismutases in malignant cells and human tumors. Free Radical Biol. Med. 2004;36:718–744. doi: 10.1016/j.freeradbiomed.2003.12.010. [DOI] [PubMed] [Google Scholar]
- 104.Nohl H., Jordan W. The metabolic fate of mitochondrial hydrogen peroxide. Eur. J. Biochem. 1980;111:203–210. doi: 10.1111/j.1432-1033.1980.tb06094.x. [DOI] [PubMed] [Google Scholar]
- 104a.Halliwell B., Gutteridge J. M. C. Biology and Medicine. Oxford, UK: Clarendon Press; 1989. Free radicals. [Google Scholar]
- 104b.Schroder E., Ponting C. P. Evidence that peroxiredoxins are novel members of the thioredoxin-fold family. Protein Science. 1998;7:2465–2468. doi: 10.1002/pro.5560071125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Chen Z., Siu B., Ho Y. S., Vincent R., Chua C. C., Hamdy R. C., Chua B. H. Overexpression of MnSOD protects against myocardial ischemia/reperfusion injury in transgenic mice. J. Mol. Cell. Cardiol. 1998;30:2281–2289. doi: 10.1006/jmcc.1998.0789. [DOI] [PubMed] [Google Scholar]
- 106.Asimakis G. K., Lick S., Patterson C. Postischemic recovery of contractile function is impaired in SOD2(+/−) but not SOD1 (+/−) mouse hearts. Circulation. 2002;105:981–986. doi: 10.1161/hc0802.104502. [DOI] [PubMed] [Google Scholar]
- 107.Ueta H., Ogura R., Sugiyama M., Kagiyama A., Shin G. O2-spin trapping on cardiac submitochondrial particles isolated from ischemic and non-ischemic myocardium. J. Mol. Cell. Cardiol. 1990;22:893–899. doi: 10.1016/0022-2828(90)90120-q. [DOI] [PubMed] [Google Scholar]
- 108.Suzuki K., Murtuza B., Sammut I. A., Latif N., Jayakumar J., Smolenski R. T., Kaneda Y., Sawa Y., Matsuda H., Yacoub M. H. Heat-shock protein 72 enhances manganese superoxide dismutase activity during myocardial ischemia-reperfusion injury, associated with mitochondrial protection and apoptosis reduction. Circulation. 2002;106:I270–276. [PubMed] [Google Scholar]
- 109.Nohl H. Generation of superoxide radicals as byproduct of cellular respiration. Ann. Biol. Clin. (Paris) 1994;52:199–204. [PubMed] [Google Scholar]
- 110.Lenaz G. The mitochondrial production of reactive oxygen species: mechanisms and implications in human pathology. IUBMB Life. 2001;52:59–64. doi: 10.1080/15216540152845957. [DOI] [PubMed] [Google Scholar]
- 111.Kushnareva Y., Murphy A. N., Andreyev A. Complex I-mediated reactive oxygen species generation: modulation by cytochrome c and NAD(P)+ oxidation-reduction state. Biochem. J. 2002;368:545–553. doi: 10.1042/BJ20021121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Becker L. B. New concepts in reactive oxygen species and cardiovascular reperfusion physiology. Cardiovasc. Res. 2004;61:461–470. doi: 10.1016/j.cardiores.2003.10.025. [DOI] [PubMed] [Google Scholar]
- 112a.Paddenberg R., Ishaq B., Goldenberg A., Faulhammer P., Rose F., Weissmann N., Braun-Dullaeus R. C., Kummer W. Essential role of complex II of the respiratory chain in hypoxia-induced ROS generation in the pulmonary vasculature. Am. J. Physiol. Lung. Cell. Mol. Physiol. 2003;284:L710–L719. doi: 10.1152/ajplung.00149.2002. [DOI] [PubMed] [Google Scholar]
- 113.Yankovskaya V., Horsefield R., Tornroth S., Luna-Chavez C., Miyoshi H., Leger C., Byrne B., Cecchini G., Iwata S. Architecture of succinate dehydrogenase and reactive oxygen species generation. Science (Washington, D.C.) 2003;299:671–672. doi: 10.1126/science.1079605. [DOI] [PubMed] [Google Scholar]
- 114.Messner K. R., Imlay J. A. Mechanism of superoxide and hydrogen peroxide formation by fumarate reductase, succinate dehydrogenase, and aspartate oxidase. J. Biol. Chem. 2002;277:42563–42571. doi: 10.1074/jbc.M204958200. [DOI] [PubMed] [Google Scholar]
- 115.Knowles R. G., Moncada S. Nitric oxide synthases in mammals. Biochem. J. 1994;298:249–258. doi: 10.1042/bj2980249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Clementim E., Brown G. C., Feelisch M., Moncada S. Persistent inhibition of cell respiration by nitric oxide: crucial role of S-nitrosylation of mitochondrial complex I and protective action of glutathione. Proc. Natl. Acad. Sci. U.S.A. 1998;95:7631–7636. doi: 10.1073/pnas.95.13.7631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Leist M., Single B., Naumann H., Fava E., Simon B., Kuhnle S., Nicotera P. Inhibition of mitochondrial ATP generation by nitric oxide switches apoptosis to necrosis. Exp. Cell. Res. 1999;249:346–353. doi: 10.1006/excr.1999.4514. [DOI] [PubMed] [Google Scholar]
- 118.Hortelano S., Dallaporta B., Zamzami N., Hirsch T., Susin S. A., Marzo I., Bosca L., Kroemer G. Nitric oxide induces apoptosis via triggering mitochondrial permeability transition. FEBS Lett. 1999;410:373–377. doi: 10.1016/s0014-5793(97)00623-6. [DOI] [PubMed] [Google Scholar]
- 119.Cleeter M. W., Cooper J. M., Darley-Usmar V. M., Moncada S., Schapira A. H. Reversible inhibition of cytochrome c oxidase, the terminal enzyme of the mitochondrial respiratory chain, by nitric oxide. Implications for neurodegenerative diseases. FEBS Lett. 1994;345:50–54. doi: 10.1016/0014-5793(94)00424-2. [DOI] [PubMed] [Google Scholar]
- 120.Schopfner F., Riobo' N., Carreras M. C., Alvarez B., Radi R., Boveris A., Cadenas E., Poderoso J. J. Oxidation of ubiquinol by peroxynitrite: implications for protection of mitochondria against nitrosative damage. Biochem. J. 2000;349:36–42. doi: 10.1042/0264-6021:3490035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Kim Y. M., Bombeck C. A., Billiar T. R. Nitric oxide as a bifunctional regulator of apoptosis. Circ. Res. 1999;84:253–256. doi: 10.1161/01.res.84.3.253. [DOI] [PubMed] [Google Scholar]
- 122.Liu L., Stamler J. S. NO: an inhibitor of cell death. Cell Death Differ. 1999;6:937–942. doi: 10.1038/sj.cdd.4400578. [DOI] [PubMed] [Google Scholar]
- 123.Rakhit R. D., Mojet M. H., Marber M. S., Duchen M. R. Mitochondria as targets for nitric oxide-induced protection during simulated ischemia and reoxygenation in isolated neonatal cardiomyocytes. Circulation. 2001;103:2617–2623. doi: 10.1161/01.cir.103.21.2617. [DOI] [PubMed] [Google Scholar]
- 124.Brunner F., Maier R., Andrew P., Wolkart G., Zechner R., Mayer B. Attenuation of myocardial ischemia/reperfusion injury in mice with myocyte-specific overexpression of eNOS. Cardiovasc. Res. 2003;57:55–62. doi: 10.1016/s0008-6363(02)00649-1. [DOI] [PubMed] [Google Scholar]
- 125.Beltran B., Quintero M., Garcia-Zaragoza E., O'Connor E., Esplugues J. V., Moncada S. Inhibition of mitochondrial respiration by endogenous nitric oxide: a critical step in Fas signaling. Proc. Natl. Acad. Sci. U.S.A. 2002;99:8892–8897. doi: 10.1073/pnas.092259799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.French S., Giulivi C., Balaban R. S. Nitric oxide synthase in porcine heart mitochondria: evidence for low physiological activity. Am. J. Physiol. Heart Circ. Physiol. 2001;280:H2863–H2867. doi: 10.1152/ajpheart.2001.280.6.H2863. [DOI] [PubMed] [Google Scholar]
- 127.Tiravanti E., Samouilov A., Zweiler J. L. Nitrosyl-heme complexes are formed in the ischaemic heart: evidence of nitrite-derived nitric oxide formation, storage, and signalling in post-ischemic tissues. J. Biol. Chem. 2004;279:11065–11073. doi: 10.1074/jbc.M311908200. [DOI] [PubMed] [Google Scholar]
- 128.Lacza Z., Puskar M., Figueroa J. P., Zhang J., Rajapakse N., Busija D. W. Mitochondrial nitric oxide synthase is constitutively active and is functionally upregulated in hypoxia. Free Radical Biol. Med. 2001;31:1609–1615. doi: 10.1016/s0891-5849(01)00754-7. [DOI] [PubMed] [Google Scholar]
- 129.Harris D. A., Das A. M. Control of mitochondrial ATP synthesis in the heart. Biochem. J. 1991;280:561–573. doi: 10.1042/bj2800561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Green D. W., Grover G. J. The IF1 inhibitor protein of the mitochondrial F1Fo-ATPase. Biochim. Biophys. Acta. 2000;1458:343–355. doi: 10.1016/s0005-2728(00)00085-2. [DOI] [PubMed] [Google Scholar]
- 131.Territo P. R., Mootha V. K., French S. A., Balaban R. S. Ca2+ activation of heart mitochondrial oxidative phosphorylation: role of the F0F1-ATPase. Am. J. Physiol. 2000;278:C423–C435. doi: 10.1152/ajpcell.2000.278.2.C423. [DOI] [PubMed] [Google Scholar]
- 132.Ylitalo K., Ala Rami A., Vuorinen K., Peuhkurinen K., Lepojarvi M., Kaukoranta P., Kiviluoma K., Hassinen I. Reversible ischaemic inhibition of F1F0-ATPase in rat and human myocardium. Biochim. Biophys. Acta. 2001;1504:329–339. doi: 10.1016/s0005-2728(00)00261-9. [DOI] [PubMed] [Google Scholar]
- 133.Cortassa S., Aon M. A., Marban E., Winslow R. L., O'Rourke B. An integrated model of cardiac mitochondrial energy metabolism and calcium dynamics. Biophys. J. 2003;84:2734–2755. doi: 10.1016/S0006-3495(03)75079-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Rouslin W. The mitochondrial adenosine 5′-triphosphatase in slow and fast heart rate hearts. Am. J. Physiol. 1987;252:H622–H627. doi: 10.1152/ajpheart.1987.252.3.H622. [DOI] [PubMed] [Google Scholar]
- 135.Rouslin W., Broge C. W. Mechanisms of ATP conservation during ischemia in slow and fast heart rate hearts. Am. J. Physiol. 1993;264:C209–C216. doi: 10.1152/ajpcell.1993.264.1.C209. [DOI] [PubMed] [Google Scholar]
- 136.Vander Heide R. S., Hill M. L., Reimer K. A., Jennings R. B. Effect of reversible ischemia on the activity of the mitochondrial ATPase: relationship to ischemic preconditioning. J. Mol. Cell. Cardiol. 1996;28:103–112. doi: 10.1006/jmcc.1996.0010. [DOI] [PubMed] [Google Scholar]
- 137.Scholz T. D., Balaban R. S. Mitochondrial F1-ATPase activity of canine myocardium: effects of hypoxia and stimulation. Am. J. Physiol. 1994;266:H2396–H2403. doi: 10.1152/ajpheart.1994.266.6.H2396. [DOI] [PubMed] [Google Scholar]
- 138.Power J., Cross R. L., Harris D. A. Interaction of F1-ATPase, from ox heart mitochondria with its naturally occurring inhibitor protein. Studies using radio-iodinated inhibitor protein. Biochim. Biophys. Acta. 1983;724:128–141. doi: 10.1016/0005-2728(83)90034-8. [DOI] [PubMed] [Google Scholar]
- 139.Bosetti F., Yu G., Zucchi R., Ronca-Testoni S., Solaini G. Myocardial ischemic preconditioning, and mitochondrial F1Fo-ATPase activity. Mol. Cell. Biochem. 2000;215:31–38. doi: 10.1023/a:1026558922596. [DOI] [PubMed] [Google Scholar]
- 140.Di Pancrazio F., Mavelli I., Isola M., Losano G., Pagliaro P., Harris D. A., Lippe G. In vitro and in vivo studies of F0F1ATP synthase regulation by inhibitor protein IF1 in goat heart. Biochim. Biophys. Acta. 2004;1659:52–62. doi: 10.1016/j.bbabio.2004.07.009. [DOI] [PubMed] [Google Scholar]
- 141.Murry C. E., Richard V. J., Reimer K. A., Jennings R. B. Ischemic preconditioning slows energy metabolism and delays ultrastructural damage during a sustained ischemic episode. Circ. Res. 1990;66:913–931. doi: 10.1161/01.res.66.4.913. [DOI] [PubMed] [Google Scholar]
- 142.Hassinen I. E., Vuorinen K. H., Ylitalo K., Ala Rami A. Role of cellular energetics in ischemia-reperfusion and ischemic preconditioning of myocardium. Mol. Cell. Biochem. 1998;184:393–400. [PubMed] [Google Scholar]
- 143.Green D. W., Murray H. N., Sleph P. G., Wang F. L., Baird A. J., Rogers W. L., Grover G. J. Preconditioning in rat hearts is independent of mitochondrial F1Fo-ATPase inhibition. Am. J. Physiol. 1998;274:H90–H97. doi: 10.1152/ajpheart.1998.274.1.H90. [DOI] [PubMed] [Google Scholar]
- 144.Ala-Rami A., Ylitalo K. V., Hassinen I. E. Ischemic preconditioning and a mitochondrial KATP channel opener both produce cardioprotection accompanied by F1Fo-ATPase inhibition in early ischemia. Basic Res. Cardiol. 2003;98:250–258. doi: 10.1007/s00395-003-0413-z. [DOI] [PubMed] [Google Scholar]
- 145.Walker J. E., Runswick M. J. The mitochondrial transporter superfamily. J. Bioenerg. Biomembr. 1993;25:436–446. doi: 10.1007/BF01108401. [DOI] [PubMed] [Google Scholar]
- 146.Ricquier D., Bouillaud F. The mitochondrial uncoupling protein; structural and genetic studies. Prog. Nucleic Acid Res. Mol. Biol. 1997;56:83–108. doi: 10.1016/s0079-6603(08)61003-x. [DOI] [PubMed] [Google Scholar]
- 147.Xu W., Liu Y., Wang S., McDonald T., van Eyk J. E., Sidor A., O'Rourke B. Cytoprotective role of Ca2+-activated channels of the inner mitochondrial membrane. Science (Washington, D.C.) 2002;298:1029–1033. doi: 10.1126/science.1074360. [DOI] [PubMed] [Google Scholar]
- 148.Levraut J., Iwase H., Shao Z. H., Vanden Hoek T. L., Schumacker P. T. Cell death during ischemia; relationship to mitochondrial depolarisation and ROS generation. Am. J. Physiol. 2003;284:H549–H558. doi: 10.1152/ajpheart.00708.2002. [DOI] [PubMed] [Google Scholar]
- 149.Taniguchi M., Wilson C., Hunter C. A., Pehowich D. J., Clanachan A. S., Lopaschuk G. D. Dichloroacetate improves cardiac efficiency after ischemia independent of changes in mitochondrial proton leak. Am. J. Physiol. 2001;280:H1762–H1769. doi: 10.1152/ajpheart.2001.280.4.H1762. [DOI] [PubMed] [Google Scholar]
- 150.Yamamoto S., Matsui K., Ohashi N. Protective effect of Na+/H+ exchange inhibitor, SM-20550, on impaired mitochondrial respiratory function and mitochondrial Ca2+ overload in ischemic/reperfused rat hearts. J. Cardiovasc. Pharmacol. 2002;39:569–575. doi: 10.1097/00005344-200204000-00013. [DOI] [PubMed] [Google Scholar]
- 151.Demaison L., Grynberg A. Cellular and mitochondrial energy metabolism in the stunned myocardium. Basic Res. Cardiol. 1994;89:293–307. doi: 10.1007/BF00795199. [DOI] [PubMed] [Google Scholar]
- 152.Minners J., Van Den Bos E. J., Yellon D. M., Schwalb H., Opie L. H., Sack M. N. Dinitrophenol, cyclosporin A, and trimetazidine modulate preconditioning in the isolated rat heart: support for a mitochondrial role in cardioprotection. Cardiovasc. Res. 2000;47:68–73. doi: 10.1016/s0008-6363(00)00069-9. [DOI] [PubMed] [Google Scholar]
- 153.Rodrigo G. C., Lawrence C. L., Standen N. B. Dinitrophenol pretreatment of rat ventricular myocytes protects against damage by metabolic inhibition and reperfusion. J. Mol. Cell. Cardiol. 2002;34:555–569. doi: 10.1006/jmcc.2002.1536. [DOI] [PubMed] [Google Scholar]
- 154.Bienengraeber M., Ozcan C., Terzic A. Stable transfection of UCP1 confers resistance to hypoxia/reoxygenation in a heart-derived cell line. J. Mol. Cell. Cardiol. 2003;35:861–865. doi: 10.1016/s0022-2828(03)00147-0. [DOI] [PubMed] [Google Scholar]
- 155.Hoerter J., Gonzalez-Barroso M., Couplan E., Mateo P., Gelly C., Cassard-Doulcier A. M., Diolez P., Bouillaud F. Mitochondrial UCP1 expressed in the heart of transgenic mice protects against ischemic-reperfusion damage. Circulation. 2004;110:528–533. doi: 10.1161/01.CIR.0000137824.30476.0E. [DOI] [PubMed] [Google Scholar]
- 156.Miyata H., Silverman H. S., Sollott S. J., Lakatta E. G., Stern M. D., Hansford R. G. Measurement of mitochondrial free Ca2+ concentration in living single rat cardiac myocytes. Am. J. Physiol. 1991;261:H1123–H1134. doi: 10.1152/ajpheart.1991.261.4.H1123. [DOI] [PubMed] [Google Scholar]
- 157.McCormack J. G., Halestrap A. P., Denton R. M. Role of calcium ions in regulation of mammalian intramitochondrial metabolism. Physiol. Rev. 1990;70:391–425. doi: 10.1152/physrev.1990.70.2.391. [DOI] [PubMed] [Google Scholar]
- 158.Rizzuto R., Pinton P., Brini M., Chiesa A., Filippin L., Pozzan T. Mitochondria as biosensors of calcium microdomains. Cell Calcium. 1999;26:193–199. doi: 10.1054/ceca.1999.0076. [DOI] [PubMed] [Google Scholar]
- 159.Allard M. F., Flint J. D., English J. C., Henning S. L., Salamanca M. C., Kamimura C. T., English D. R. Calcium overload during reperfusion is accelerated in isolated hypertrophied rat hearts. J. Mol. Cell. Cardiol. 1994;26:1551–1563. doi: 10.1006/jmcc.1994.1175. [DOI] [PubMed] [Google Scholar]
- 160.Varadarajan S. G., An J., Novalija E., Smart S. C., Stowe D. F. Changes in [Na+]i, compartmental [Ca2+], and NADH with dysfunction after global ischemia in intact hearts. Am. J. Physiol. 2001;280:H280–H293. doi: 10.1152/ajpheart.2001.280.1.H280. [DOI] [PubMed] [Google Scholar]
- 161.Ferrari R., Di Lisa F., Raddino R., Visioli O. The effects of ruthenium red on mitochondrial function during post-ischemic reperfusion. J. Mol. Cell. Cardiol. 1982;14:737–740. doi: 10.1016/0022-2828(82)90186-9. [DOI] [PubMed] [Google Scholar]
- 162.Benzi R. H., Lerch R. Dissociation between contractile function and oxidative metabolism in postischemic myocardium. Attenuation by ruthenium red administered during reperfusion. Circ. Res. 1992;71:567–576. doi: 10.1161/01.res.71.3.567. [DOI] [PubMed] [Google Scholar]
- 163.Iwai T., Tanonaka K., Inoue R., Kasahara S., Kamo N., Takeo S. Mitochondrial damage during ischemia determines post-ischemic contractile dysfunction in perfused rat heart. J. Mol. Cell. Cardiol. 2002;34:725–738. doi: 10.1006/jmcc.2002.2002. [DOI] [PubMed] [Google Scholar]
- 164.Tani M., Neely J. R. Mechanisms of reduced reperfusion injury by low Ca2+ and/or high K+ Am. J. Physiol. 1990;258:H1025–H1031. doi: 10.1152/ajpheart.1990.258.4.H1025. [DOI] [PubMed] [Google Scholar]
- 165.Arieli Y., Gursahani H., Eaton M., Hernandez L. A., Schaefer S. Gender modulation of Ca2+ uptake in cardiac mitochondria. J. Mol. Cell. Cardiol. 2004;37:507–513. doi: 10.1016/j.yjmcc.2004.04.023. [DOI] [PubMed] [Google Scholar]
- 166.Garlid K. D., Paucek P., Yarovoy V., Sun X., Schindler P. A. The mitochondrial KATP channel as a receptor for potassium channel openers. J. Biol. Chem. 1996;271:8796–8799. doi: 10.1074/jbc.271.15.8796. [DOI] [PubMed] [Google Scholar]
- 167.Seharaseyon J., Ohler A., Sasaki N., Fraser H., Sato T., Johns D. C., O'Rourke B., Marbán E. Molecular composition of mitochondrial ATP-sensitive potassium channels probed by viral kir gene transfer. J. Mol. Cell. Cardiol. 2000;32:1923–1930. doi: 10.1006/jmcc.2000.1226. [DOI] [PubMed] [Google Scholar]
- 168.Kowaltowski A. J., Seetharaman S., Paucek P., Garlid K. D. Bioenergetic consequences of opening the ATP-sensitive K+ channel of heart mitochondria. Am. J. Physiol. 2001;280:H649–H657. doi: 10.1152/ajpheart.2001.280.2.H649. [DOI] [PubMed] [Google Scholar]
- 169.Garlid K. D., Paucek P., Yarovoy V., Murray H. N., Darbenzio R. B., D'Alonzo A. J., Lodge N. J., Smith M. A., Grover G. J. Cardioprotective effect of diazoxide and its interaction with mitochondrial ATP-sensitive K+ channels. Possible mechanism of cardioprotection. Circ. Res. 1997;81:1072–1082. doi: 10.1161/01.res.81.6.1072. [DOI] [PubMed] [Google Scholar]
- 170.Liu Y., Sato T., Seharaseyon J., Szewczyk A., O'Rourke B., Marban E. Mitochondrial ATP-dependent potassium channels. Viable candidate effectors of ischemic preconditioning. Ann. N.Y. Acad. Sci. 1999;874:27–32. doi: 10.1111/j.1749-6632.1999.tb09222.x. [DOI] [PubMed] [Google Scholar]
- 171.Ghosh S., Standen N. B., Galinanes M. Evidence for mitochondrial KATP channels as effectors of human myocardial preconditioning. Cardiovasc. Res. 2000;45:934–940. doi: 10.1016/s0008-6363(99)00407-1. [DOI] [PubMed] [Google Scholar]
- 172.Oldenburg O., Cohen M. V., Downey J. M. Mitochondrial KATP channels in preconditioning. J. Mol. Cell. Cardiol. 2003;35:569–575. doi: 10.1016/s0022-2828(03)00115-9. [DOI] [PubMed] [Google Scholar]
- 173.Ferranti R., Da Silva M. M., Kowaltowski A. J. Mitochondrial ATP-sensitive K+ channel opening decreases reactive oxygen species generation. FEBS Lett. 2003;536:51–55. doi: 10.1016/s0014-5793(03)00007-3. [DOI] [PubMed] [Google Scholar]
- 174.O'Rourke B. Evidence for mitochondrial K+-channels and their role in cardioprotection. Circ. Res. 2004;94:420–32. doi: 10.1161/01.RES.0000117583.66950.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Askenasy N., Navon G. Intermittent ischemia: energy metabolism, cellular volume regulation, adenosine and insights into preconditioning. J. Mol. Cell. Cardiol. 1997;29:1715–1730. doi: 10.1006/jmcc.1997.0410. [DOI] [PubMed] [Google Scholar]
- 176.Schafer G., Wegener C., Portenhauser R., Bojanovski D. Diazoxide, an inhibitor of succinate oxidation. Biochem. Pharmacol. 1969;18:2678–2681. [PubMed] [Google Scholar]
- 177.Hanley P. J., Mickel M., Loffler M., Brandt U., Daut J. KATP channel-independent targets of diazoxide and 5-hydroxydecanoate in the heart. J. Physiol. 2002;542:735–741. doi: 10.1113/jphysiol.2002.023960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Contessi S., Metelli G., Mavelli I., Lippe G. Diazoxide affects the IF1 inhibitor protein binding to F1 sector of beef heart F0F1ATP synthase. Biochem. Pharmacol. 2004;67:1843–1851. doi: 10.1016/j.bcp.2004.02.001. [DOI] [PubMed] [Google Scholar]
- 179.Dos Santos P., Kowaltowski A. J., Laclau M. N., Seetharaman S., Paucek P., Boudina S., Thambo J. B., Tariosse L., Garlid K. D. Mechanisms by which opening the mitochondrial ATP-sensitive K+ channel protects the ischemic heart. Am. J. Physiol. 2002;283:H284–H295. doi: 10.1152/ajpheart.00034.2002. [DOI] [PubMed] [Google Scholar]
- 180.Ganote C. E., Armstrong S. C. Effects of CCCP-induced mitochondrial uncoupling and cyclosporin A on cell volume, cell injury and preconditioning protection of isolated rabbit cardiomyocytes. J. Mol. Cell. Cardiol. 2003;35:749–759. doi: 10.1016/s0022-2828(03)00114-7. [DOI] [PubMed] [Google Scholar]
- 181.Das M., Parker J. E., Halestrap A. P. Matrix volume measurements challenge the existence of diazoxide/glibencamide-sensitive KATP channels in rat mitochondria. J. Physiol. 2003;547:893–902. doi: 10.1113/jphysiol.2002.035006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Xu W., Liu Y., Wang S., McDonald T., Van Eyk J. E., Sidor A., O'Rourke B. Cytoprotective role of Ca2+-activated K+ channels in the cardiac inner mitochondrial membrane. Science (Washington, D.C.) 2002;298:1029–1033. doi: 10.1126/science.1074360. [DOI] [PubMed] [Google Scholar]
- 183.Pike M. M., Luo C. S., Clark M. D., Kirk K. A., Kitakaze M., Madden M. C., Cragoe E. J., Jr, Pohost G. M. NMR measurements of Na+ and cellular energy in ischemic rat heart: role of Na+/H+ exchange. Am. J. Physiol. 1993;265:H2017–H2026. doi: 10.1152/ajpheart.1993.265.6.H2017. [DOI] [PubMed] [Google Scholar]
- 184.Varadarajan S. G., An J., Novalija E., Smart S. C., Stowe D. F. Changes in [Na+]i, compartmental [Ca2+], and NADH with dysfunction after global ischemia in intact hearts. Am. J. Physiol. 2001;280:H280–H293. doi: 10.1152/ajpheart.2001.280.1.H280. [DOI] [PubMed] [Google Scholar]
- 185.Anderson S. E., Murphy E., Steenbergen C., London R. E., Cala P. M. Na-H exchange in myocardium: effects of hypoxia and acidification on Na and Ca. Am. J. Physiol. 1990;259:C940–C948. doi: 10.1152/ajpcell.1990.259.6.C940. [DOI] [PubMed] [Google Scholar]
- 186.Lazdunski M., Frelin C., Vigne P. The sodium/hydrogen exchange system in cardiac cells: its biochemical and pharmacological properties and its role in regulating internal concentrations of sodium and internal pH. J. Mol. Cell. Cardiol. 1985;17:1029–1042. doi: 10.1016/s0022-2828(85)80119-x. [DOI] [PubMed] [Google Scholar]
- 187.Van Borren M. M. G. J., Baartscheer A., Wilders R., Ravesloot J. H. NHE-1 and NBC during pseudo-ischemia/reperfusion in rabbit ventricular myocytes. J. Mol. Cell. Cardiol. 2004;37:567–577. doi: 10.1016/j.yjmcc.2004.05.017. [DOI] [PubMed] [Google Scholar]
- 188.Tani M., Neely J. R. Role of intracellular Na+ in Ca2+ overload and depressed recovery of ventricular function of reperfused ischemic rat hearts. Possible involvement of H+-Na+ and Na+-Ca2+ exchange. Circ. Res. 1989;65:1045–1056. doi: 10.1161/01.res.65.4.1045. [DOI] [PubMed] [Google Scholar]
- 189.Satoh H., Hayashi H., Noda N., Terada H., Kobayashi A., Hirano M., Yamashita Y., Yamazaki N. Regulation of [Na+]i and [Ca2+]i in guinea pig myocytes: dual loading of fluorescent indicators SBFI and fluo 3. Am. J. Physiol. 1994;266:H568–H576. doi: 10.1152/ajpheart.1994.266.2.H568. [DOI] [PubMed] [Google Scholar]
- 190.Cross H. R., Lu L., Steenbergen C., Philipson K. D., Murphy E. Overexpression of the cardiac Na+/Ca2+ exchanger increases susceptibility to ischemia/reperfusion injury in male, but not female, transgenic mice. Circ. Res. 1998;83:1215–1223. doi: 10.1161/01.res.83.12.1215. [DOI] [PubMed] [Google Scholar]
- 191.Imahashi K., Kusuoka H., Hashimoto K., Yoshioka J., Yamaguchi H., Nishimura T. Intracellular sodium accumulation during ischemia as the substrate for reperfusion injury. Circ. Res. 1999;84:1401–1406. doi: 10.1161/01.res.84.12.1401. [DOI] [PubMed] [Google Scholar]
- 192.Crompton M. The role of Ca2+ in the function and dysfunction of heart mitochondria. In: Lange G. A., editor. Calcium and the Heart. NY: Raven Press; 1990. pp. 167–198. [Google Scholar]
- 193.Crompton M., Virji S., Ward J. M. Cyclophilin-D binds strongly to complexes of the voltage-dependent anion channel and the adenine nucleotide translocase to form the permeability transition pore. Eur. J. Biochem. 1998;258:729–735. doi: 10.1046/j.1432-1327.1998.2580729.x. [DOI] [PubMed] [Google Scholar]
- 194.Graham R. M., Frazier D. P., Thompson J. W., Haliko S., Li H., Wasserlauf B. J., Spiga M. G., Bishopric N. H., Webster K. A. A unique pathway of cardiac myocyte death caused by hypoxia-acidosis. J. Exp. Biol. 2004;207:3189–3200. doi: 10.1242/jeb.01109. [DOI] [PubMed] [Google Scholar]
- 195.Baines C. P., Song C.-X., Zheng Y.-T., Wang G.-W., Zhang J., Wang O.-L., Guo Y., Bolli R., Cardwell E. M., Ping P. Protein kinase c[ϵ] interacts with and inhibits the permeability transition pore in cardiac mitochondria. Circ. Res. 2003;92:873–880. doi: 10.1161/01.RES.0000069215.36389.8D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Duchen M. R., McGuinness O., Brown L. A., Crompton M. On the involvement of a cyclosporin A sensitive mitochondrial pore in myocardial reperfusion injury. Cardiovasc. Res. 1993;27:1790–1794. doi: 10.1093/cvr/27.10.1790. [DOI] [PubMed] [Google Scholar]
- 197.Griffiths E. J., Halestrap A. P. Mitochondrial non-specific pores remain closed during cardiac ischaemia, but open upon reperfusion. Biochem. J. 1995;307:93–98. doi: 10.1042/bj3070093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Di Lisa F., Menabò R., Canton M., Barile M., Bernardi P. Opening of the mitochondrial permeability transition pore causes depletion of mitochondrial and cytosolic NAD+ and is a causative event in the death of myocytes in post-ischemic reperfusion of the heart. J. Biol. Chem. 2001;276:2571–2575. doi: 10.1074/jbc.M006825200. [DOI] [PubMed] [Google Scholar]
- 199.Halestrap A. P., Kerr P. M., Javadov S., Woodfield K. Y. Elucidating the molecular mechanism of the permeability transition pore and its role in reperfusion injury of the heart. Biochim. Biophys. Acta. 1998;1366:79–94. doi: 10.1016/s0005-2728(98)00122-4. [DOI] [PubMed] [Google Scholar]
- 200.Gottlieb R. A., Burleson K. O., Kloner R. A., Babior B. M., Engler R. L. Reperfusion injury induces apoptosis in rabbit cardiomyocytes. J. Clin. Invest. 1994;94:1621–1628. doi: 10.1172/JCI117504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Zhao Z. Q., Nakamura M., Wang N. P., Wilcox J. N., Shearer S., Ronson R. S., Guyton R. A., Vinten Johansen J. Reperfusion induces myocardial apoptotic cell death. Cardiovasc. Res. 2000;45:651–660. doi: 10.1016/s0008-6363(99)00354-5. [DOI] [PubMed] [Google Scholar]
- 202.Eefting F., Rensing B., Wigman J., Pannekoek W. J., Liu W. M., Cramer M. J., Lips D. J., Doevendans P. A. Role of apoptosis in reperfusion injury. Cardiovasc. Res. 2004;61:414–426. doi: 10.1016/j.cardiores.2003.12.023. [DOI] [PubMed] [Google Scholar]
- 203.Borutaite V., Jekabsone A., Morkuniene R., Brown G. C. Inhibition of mitochondrial permeability transition prevents mitochondrial dysfunction, cytochrome c release and apoptosis induced by heart ischemia. J. Mol. Cell. Cardiol. 2003;35:357–366. doi: 10.1016/s0022-2828(03)00005-1. [DOI] [PubMed] [Google Scholar]
- 204.Zamzami N., Marchetti P., Castedo M., Hirsch T., Susin S. A., Masse B., Kroemer G. Inhibitors of permeability transition interfere with the disruption of the mitochondrial transmembrane potential during apoptosis. FEBS. Lett. 1996;384:53–57. doi: 10.1016/0014-5793(96)00280-3. [DOI] [PubMed] [Google Scholar]
- 205.Visioli F., Borsani L., Galli C. Diet and prevention of coronary heart disease: the potential role of phytochemicals. Cardiovasc. Res. 2000;47:419–425. doi: 10.1016/s0008-6363(00)00053-5. [DOI] [PubMed] [Google Scholar]
- 206.Varela A. M., Prendes M. G., Testoni G., Vazquez N., Astudilla C., Cerruti S., Savino E. A. Influence of fasting on the effects of ischemic preconditioning in the ischemic-reperfused rat heart. Arch. Physiol. Biochem. 2002;110:189–196. doi: 10.1076/apab.110.3.189.8291. [DOI] [PubMed] [Google Scholar]
- 207.Broderick T. L., Belke T., Driedzic W. R. Effects of chronic caloric restriction on mitochondrial respiration in the ischemic reperfused rat heart. Mol. Cell. Biochem. 2002;233:119–125. doi: 10.1023/a:1015506327849. [DOI] [PubMed] [Google Scholar]
- 208.Murry C. E., Jennings R. B., Reimer K. A. Preconditioning with ischemia: a delay of lethal cell injury in ischemic myocardium. Circulation. 1986;74:1124–1136. doi: 10.1161/01.cir.74.5.1124. [DOI] [PubMed] [Google Scholar]
- 209.Henry P. D., Sobel B. E., Braunwald E. Protection of hypoxic guinea pig hearts with glucose and insulin. Am. J. Physiol. 1974;226:309–313. doi: 10.1152/ajplegacy.1974.226.2.309. [DOI] [PubMed] [Google Scholar]
- 210.Ramasamy R., Hwang Y. C., Whang J., Bergmann S. R. Protection of ischemic hearts by high glucose is mediated, in part, by GLUT-4. Am. J. Physiol. Heart Circ. Physiol. 2001;281:H290–H297. doi: 10.1152/ajpheart.2001.281.1.H290. [DOI] [PubMed] [Google Scholar]
- 211.Stanley W. C., Lopaschuk G. D., Hall J. L., McCormack J. G. Regulation of myocardial carbohydrate metabolism under normal and ischemic conditions. Potential for pharmacological interventions. Cardiovasc. Res. 1997;33:243–257. doi: 10.1016/s0008-6363(96)00245-3. [DOI] [PubMed] [Google Scholar]
- 211.Bunger R., Mallet R. T., Hartman D. A. Pyruvate-enhanced phosphorylation potential and inotropism in normoxic and postischemic isolated working heart. Near-complete prevention of reperfusion contractile failure. Eur. J. Biochem. 1989;189:221–233. doi: 10.1111/j.1432-1033.1989.tb14637.x. [DOI] [PubMed] [Google Scholar]
- 213.Cavallini L., Valente M., Rigobello M. P. The protective action of pyruvate on recovery of ischemic rat heart: comparison with other oxidizable substrates. J. Mol. Cell. Cardiol. 1990;22:143–154. doi: 10.1016/0022-2828(90)91111-j. [DOI] [PubMed] [Google Scholar]
- 214.Fantini E., Demaison L., Sentex E., Grynberg A., Athias P. Some biochemical aspects of the protective effect of trimetazidine on rat cardiomyocytes during hypoxia and reoxygenation. J. Mol. Cell. Cardiol. 1994;26:949–958. doi: 10.1006/jmcc.1994.1116. [DOI] [PubMed] [Google Scholar]
- 215.Higgins A. J., Morville M., Burges R. A., Blackburn K. J. Mechanism of action of oxfenicine on muscle metabolism. Biochem. Biophys. Res. Commun. 1981;100:291–296. doi: 10.1016/s0006-291x(81)80095-2. [DOI] [PubMed] [Google Scholar]
- 216.Chandler M. P., Chavez P. N., McElfresh T. A., Huang H., Harmon C. S., Stanley W. C. Partial inhibition of fatty acid oxidation increases regional contractile power and efficiency during demand-induced ischemia. Cardiovasc. Res. 2003;59:143–151. doi: 10.1016/s0008-6363(03)00327-4. [DOI] [PubMed] [Google Scholar]
- 217.Dyck J. R., Lopaschuk G. D. Malonyl CoA control of fatty acid oxidation in the ischemic heart. J. Mol. Cell. Cardiol. 2002;34:1099–1109. doi: 10.1006/jmcc.2002.2060. [DOI] [PubMed] [Google Scholar]
- 218.Wargovich T. J., MacDonald R. G., Hill J. A., Feldman R. L., Stacpoole P. W., Pepine C. J. Myocardial metabolic and hemodynamic effects of dichloroacetate in coronary artery disease. Am. J. Cardiol. 1988;61:65–70. doi: 10.1016/0002-9149(88)91306-9. [DOI] [PubMed] [Google Scholar]
- 219.Broderick T. L., Quinney H. A., Lopaschuk G. D. Carnitine stimulation of glucose oxidation in the fatty acid perfused isolated working rat heart. J. Biol. Chem. 1992;267:3758–3763. [PubMed] [Google Scholar]
- 220.Lango R., Smolenski R. T., Narkiewicz M., Suchorzewska J., Lysiak-Szydlowska W. Influence of L-carnitine and its derivatives on myocardial metabolism and function in ischemic heart disease and during cardiopulmonary bypass. Cardiovasc. Res. 2001;51:21–29. doi: 10.1016/s0008-6363(01)00313-3. [DOI] [PubMed] [Google Scholar]
- 221.Rowe M. J., Dolder M. A., Kirby B. J., Oliver M. F. Effect of a nicotinic-acid analogue on raised plasma-free-fatty-acids after acute myocardial infarction. Lancet. 1973;2:814–818. doi: 10.1016/s0140-6736(73)90858-1. [DOI] [PubMed] [Google Scholar]
- 222.Park J. W., Chun Y. S., Kim Y. H., Kim C. H., Kim M. S. Ischemic preconditioning reduces Op6 generation and prevents respiratory impairment in the mitochondria of post-ischemic reperfused heart of rat. Life Sci. 1997;60:2207–2219. doi: 10.1016/s0024-3205(97)00236-1. [DOI] [PubMed] [Google Scholar]
- 223.Elz J. S., Nayler W. G. Calcium gain during postischemic reperfusion. The effect of 2,4-dinitrophenol. Am. J. Pathol. 1988;131:137–145. [PMC free article] [PubMed] [Google Scholar]
- 224.Rouslin W., Erickson J. L., Solaro R. J. Effects of oligomycin and acidosis on rates of ATP depletion in ischemic heart muscle. Am. J. Physiol. 1986;250:H503–H508. doi: 10.1152/ajpheart.1986.250.3.H503. [DOI] [PubMed] [Google Scholar]
- 225.Stone D., Darley-Usmar V., Smith D. R., O'Leary V. Hypoxia-reoxygenation induced increase in cellular Ca2+ in myocytes and perfused hearts: the role of mitochondria. J. Mol. Cell. Cardiol. 1989;21:963–973. doi: 10.1016/0022-2828(89)90795-5. [DOI] [PubMed] [Google Scholar]
- 226.Grover G. J., Dzwonczyk S., Sleph P. G. Ruthenium red improves postischemic contractile function in isolated rat hearts. J. Cardiovasc. Pharmacol. 1990;16:783–789. doi: 10.1097/00005344-199011000-00014. [DOI] [PubMed] [Google Scholar]
- 227.Ferrari R., Curello S., Ceconi C., Cargnoni A., Pasini E., Visioli O. Cardioprotection by nisoldipine: role of timing of administration. Eur. Heart J. 1993;14:1258–1272. doi: 10.1093/eurheartj/14.9.1258. [DOI] [PubMed] [Google Scholar]
- 228.Kloner R. A., Braunwald E. Effects of calcium antagonists on infarcting myocardium. Am. J. Cardiol. 1987;59:B84–B94. doi: 10.1016/0002-9149(87)90087-7. [DOI] [PubMed] [Google Scholar]
- 229.Massoudy P., Becker B. F., Seligmann C., Gerlach E. Preischemic as well as postischemic application of a calcium antagonist affords cardioprotection in the isolated guinea pig heart. Cardiovasc. Res. 1995;29:577–582. [PubMed] [Google Scholar]
- 230.Ladilov Y. V., Siegmund B., Piper H. M. Protection of reoxygenated cardiomyocytes against hypercontracture by inhibition of Na+/H+ exchange. Am. J. Physiol. 1995;268:H1531–H1539. doi: 10.1152/ajpheart.1995.268.4.H1531. [DOI] [PubMed] [Google Scholar]
- 231.Garcia-Dorado M. A. G., Barrabés J. A., Ruiz-Meana M., Solares J., Lidon R. M., Blanco J., Puigfel Y. H., Michael-Piper H. M., Soler-Soler J. Prevention of ischemic rigor contracture during coronary occlusion by inhibition of Na+-H+ exchange. Cardiovasc. Res. 1995;37:80–89. doi: 10.1016/s0008-6363(97)00106-5. [DOI] [PubMed] [Google Scholar]
- 232.Philipson K. D., Nicholls D. A. Sodium-calcium exchange: a molecular perspective. Annu. Rev. Physiol. 2000;62:111–133. doi: 10.1146/annurev.physiol.62.1.111. [DOI] [PubMed] [Google Scholar]
- 233.Kroner A., Seitelberger R., Schirnhofer J., Bernecker O., Mallinger R., Hallstrom S., Ploner M., Podesser B. K. Diltiazem during reperfusion preserves high energy phosphates by protection of mitochondrial integrity. Eur. J. Cardiothorac. Surg. 2002;21:224–231. doi: 10.1016/s1010-7940(01)01110-1. [DOI] [PubMed] [Google Scholar]
- 234.Takahashi K., Takahashi T., Suzuki T., Onishi M., Tanaka Y., Hamano Takahashi A., Ota T., Kameo K., Matsuda T., Baba A. Protective effects of SEA0400, a novel and selective inhibitor of the Na+/Ca2+ exchanger, on myocardial ischemia-reperfusion injuries. Eur. J. Pharmacol. 2003;458:155–162. doi: 10.1016/s0014-2999(02)02732-2. [DOI] [PubMed] [Google Scholar]
- 235.Nazareth W., Yafei N., Crompton M. Inhibition of anoxia-induced injury in heart myocytes by cyclosporin A. J. Mol. Cell. Cardiol. 1991;23:1351–1354. doi: 10.1016/0022-2828(91)90181-k. [DOI] [PubMed] [Google Scholar]
- 236.Reimer K. A., Murry C. E., Richard V. J. The role of neutrophils and free radicals in the ischemic-reperfused heart: why the confusion and controversy? J. Mol. Cell. Cardiol. 1989;21:1225–1239. doi: 10.1016/0022-2828(89)90669-x. [DOI] [PubMed] [Google Scholar]
- 237.Visioli F., Keaney J. F., Halliwell B. Antioxidants and cardiovascular disease: panaceas or tonics for tired sheep? Cardiovasc. Res. 2000;47:409–410. doi: 10.1016/s0008-6363(00)00156-5. [DOI] [PubMed] [Google Scholar]
- 238.Dhalla N. S., Elmosheli A. B., Hata T., Makino N. Status of myocardial antioxidants in ischemia-reperfusion injury. Cardiovasc. Res. 2000;47:446–456. doi: 10.1016/s0008-6363(00)00078-x. [DOI] [PubMed] [Google Scholar]
- 239.Hung L. M., Chen J. K., Huang S. S., Lee R. S., Su M. J. Cardioprotective effect of resveratrol, a natural antioxidant derived from grapes. Cardiovasc. Res. 2000;47:549–555. doi: 10.1016/s0008-6363(00)00102-4. [DOI] [PubMed] [Google Scholar]
- 240.Cuzzocrea S., Mazzon E., Costantino G., Serraino I., De Sarro A., Caputi A. Effects of n-acetylcysteine in a rat model of ischemia and reperfusion injury. Cardiovasc. Res. 2000;47:537–548. doi: 10.1016/s0008-6363(00)00018-3. [DOI] [PubMed] [Google Scholar]
- 240a.Marczin N., El-Habashi N., Hoare G. S., Bundy R. E., Yacoub M. Antioxidants in myocardial ischaemia-reperfusion injury: therapeutic potential and basic mechanisms. Arch. Biochem. Biophys. 2003;420:222–236. doi: 10.1016/j.abb.2003.08.037. [DOI] [PubMed] [Google Scholar]
- 240b.Bandyopadhyay D., Chattopadhyay A., Ghosh G., Datta A. G. Oxidative stress-induced ischemic heart disease: protection by antioxidants. Curr. Med. Chem. 2004;11:369–387. doi: 10.2174/0929867043456016. [DOI] [PubMed] [Google Scholar]
- 241.Granville D. J., Tashakkor B., Takeuchi C., Gustafsson A. B., Huang C., Sayen M. R., Wentworth P., Yeager M., Jr, Gottlieb R. A. Reduction of ischemia and reperfusion-induced myocardial damage by cytochrome P450 inhibitors. Proc. Natl. Acad. Sci. U.S.A. 2004;101:1321–1326. doi: 10.1073/pnas.0308185100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Riess M. L., Camara A. K., Chen Q., Novalija E., Rhodes S. S., Stowe D. F. Altered NADH and improved function by anesthetic and ischemic preconditioning in guinea pig intact hearts. Am. J. Physiol. Heart Circ. Physiol. 2002;283:H53–H60. doi: 10.1152/ajpheart.01057.2001. [DOI] [PubMed] [Google Scholar]
- 243.Williams S. D., Gottlieb R. A. Inhibition of mitochondrial calcium-independent phospholipase A2 (iPLA2) attenuates mitochondrial phospholipid loss and is cardioprotective. Biochem. J. 2002;362:23–32. doi: 10.1042/0264-6021:3620023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Yellon D. M., Downey J. M. Preconditioning the myocardium: from cellular physiology to clinical cardiology. Physiol. Rev. 2003;83:1113–1151. doi: 10.1152/physrev.00009.2003. [DOI] [PubMed] [Google Scholar]
- 245.Vander-Heide R. S., Reimer K. A., Jennings R. B. Adenosine slows ischemic metabolism in canine myocardium in vitro: relationship to ischemic preconditioning. Cardiovasc. Res. 1993;27:669–673. doi: 10.1093/cvr/27.4.669. [DOI] [PubMed] [Google Scholar]
- 246.Peart J., Willems L., Headrick J. P. Receptor and non-receptor-dependent mechanisms of cardioprotection with adenosine. Am. J. Physiol. Heart Circ. Physiol. 2003;284:H519–H527. doi: 10.1152/ajpheart.00717.2002. [DOI] [PubMed] [Google Scholar]
- 247.Holden J. E., Stone C. K., Clark C. M., Brown W. D., Nickles R. J., Stanley C., Hochachka P. W. Enhanced cardiac metabolism of plasma glucose in high-altitude natives: adaptation against chronic hypoxia. J. Appl. Physiol. 1995;79:222–228. doi: 10.1152/jappl.1995.79.1.222. [DOI] [PubMed] [Google Scholar]
- 248.Opie L. H., Bruyneel K., Owen P. Effect of glucose, insulin and potassium infusion on tissue metabolic changes within first hour of myocardial infarction in the baboon. Circulation. 1975;52:49–57. doi: 10.1161/01.cir.52.1.49. [DOI] [PubMed] [Google Scholar]
- 249.Scheuer J., Stezoski S. W. Protective role of increased myocardial glycogen stores in cardiac anoxia in the rat. Circ. Res. 1970;27:835–849. doi: 10.1161/01.res.27.5.835. [DOI] [PubMed] [Google Scholar]
- 250.Henden T., Aasum E., Folkow L., Mjos O. D., Lathrop D. A., Larsen T. S. Endogenous glycogen prevents Ca2+ overload and hypercontracture in harp seal myocardial cells during simulated ischemia. J. Mol. Cell. Cardiol. 2004;37:43–50. doi: 10.1016/j.yjmcc.2004.03.013. [DOI] [PubMed] [Google Scholar]
- 251.Depre C., Vanoverschelde J. L., Taegtmeyer H. Glucose for the heart. Circulation. 1999;99:578–588. doi: 10.1161/01.cir.99.4.578. [DOI] [PubMed] [Google Scholar]
- 252.Schneider C. A., Taegtmeyer H. Fasting in vivo delays myocardial cell damage after brief periods of ischemia in the isolated working rat heart. Circ. Res. 1991;68:1045–1050. doi: 10.1161/01.res.68.4.1045. [DOI] [PubMed] [Google Scholar]
- 253.Gao F., Gao E., Yue T. L., Ohlstein E. H., Lopez B. L., Christopher T. A. Nitric oxide mediates the anti-apoptotic effect of insulin in myocardial ischemia-reperfusion: the roles of PI3-kinase, Akt, and endothelial nitric oxide synthase phosphorylation. Circulation. 2002;105:497–502. doi: 10.1161/01.cir.0000012529.00367.0f. [DOI] [PubMed] [Google Scholar]
- 254.Wolff A. A., Rotmensch H. H., Stanley W. C., Ferrari R. Metabolic approaches to the treatment of ischaemic heart disease: the clinicians' perspective. Heart Fail. Rev. 2002;7:187–203. doi: 10.1023/a:1015384710373. [DOI] [PubMed] [Google Scholar]
- 255.Weinberg J. M., Venkatachalam M. A., Roeser N. F., Nissim I. Mitochondrial dysfunction during hypoxia/reoxygenation and its correction by anaerobic metabolism of citric acid cycle intermediates. Proc. Natl. Acad. Sci. U.S.A. 2000;97:2826–2831. doi: 10.1073/pnas.97.6.2826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Scarabelli T. M., Pasini E., Stephanou A., Chen-Scarabelli C., Saravolatz L., Knight R. A., Latchman D. S., Gardin J. M. Nutritional supplementation with mixed essential amino acids enhances myocyte survival, preserving mitochondrial functional capacity during ischemia-reperfusion injury. Am. J. Cardiol. 2004;93:A35–A40. doi: 10.1016/j.amjcard.2003.11.008. [DOI] [PubMed] [Google Scholar]
- 257.Ledesma A., De Lacoba M. G., Rial E. The mitochondrial uncoupling proteins. Genome Biol. 2002;3:reviews 3015.1–3015.9. doi: 10.1186/gb-2002-3-12-reviews3015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Teshima Y., Akao M., Jones S. P., Marban E. Cariporide (HOE642), a selective Na+-H+ exchange inhibitor inhibits the mitochondrial death pathway. Circulation. 2003;108:2275–2281. doi: 10.1161/01.CIR.0000093277.20968.C7. [DOI] [PubMed] [Google Scholar]
- 259.Van Eyk J. E., Powers F., Law W., Larue C., Hodges R. S., Solaro R. J. Effects of endogenous protein as protectors of myocardial injury by ischemia and reperfusion. Circ. Res. 1998;82:261–271. doi: 10.1161/01.res.82.2.261. [DOI] [PubMed] [Google Scholar]
- 260.Lau S., Patnaik N., Sayen M. R., Mestril R. Simultaneous overexpression of two stress proteins in rat cardiomyocytes and myogenic cells confers protection against ischemia-induced injury. Circulation. 1997;96:2287–2294. doi: 10.1161/01.cir.96.7.2287. [DOI] [PubMed] [Google Scholar]
- 261.Lin K. M., Lin B., Lian I. Y., Mestril R., Scheffler I. E., Dillmann W. H. Combined and individual mitochondrial HSP60 and HSP10 expression in cardiac myocytes protects mitochondrial function and prevents apoptotic cell deaths induced by simulated ischemia-reoxygenation. Circulation. 2001;103:1787–1792. doi: 10.1161/01.cir.103.13.1787. [DOI] [PubMed] [Google Scholar]
- 262.Felts S. J., Owen B. A., Nguyen P., Trepel J., Donner D. B., Toft D. O. The hsp90-related protein TRAP1 is a mitochondrial protein with distinct functional properties. J. Biol. Chem. 2000;275:3305–3312. doi: 10.1074/jbc.275.5.3305. [DOI] [PubMed] [Google Scholar]
- 263.Kang P. M., Haunstetter A., Aoki H., Usheva A., Izumo S. Morphological and molecular characterization of adult cardiomyocyte apoptosis during hypoxia and reoxygenation. Circ. Res. 2000;87:118–125. doi: 10.1161/01.res.87.2.118. [DOI] [PubMed] [Google Scholar]
- 264.Kubasiak L. A., Hernandez O. M., Bishopric N. H., Webster K. A. Hypoxia and acidosis activate cardiac myocyte death through the Bcl-2 family protein BNIP3. Proc. Natl. Acad. Sci. U.S.A. 2002;99:12825–12830. doi: 10.1073/pnas.202474099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Gustafsson A. B., Tsai J. G., Logue S. E., Crow M. T., Gottlieb A. Apoptosis repressor with caspase recruitment domain protects against cell death by interfering with Bax activation. J. Biol. Chem. 2004;279:21233–21238. doi: 10.1074/jbc.M400695200. [DOI] [PubMed] [Google Scholar]
- 266.Cabezon E., Montgomery M. G., Leslie A. G., Walker J. E. The structure of bovine F1-ATPase in complex with its regulatory protein IF1. Nat. Struct. Biol. 2003;10:744–750. doi: 10.1038/nsb966. [DOI] [PubMed] [Google Scholar]
- 267.Lopaschuk G. D., Opie L. H. Introduction to JMCC Symposium on myocardial energy metabolism in health and disease. J. Mol. Cell. Cardiol. 2002;34:1075–1076. doi: 10.1006/jmcc.2002.2070. [DOI] [PubMed] [Google Scholar]